
Abstract
Aims: The Valve Academic Research Consortium (VARC) recommendations were devised to standardise clinical endpoint definitions which best reflect the safety and efficacy of transcatheter aortic valve implantation (TAVI). The categorisation of vascular complications (VC) is greatly affected by the definition, but the impact of its change is unclear. We sought to compare VC between VARC-1 and VARC-2 definitions as a predictor for survival.
Methods and results: A series of 376 patients undergoing TAVI by the transfemoral or transapical approach was studied. We defined VC according to VARC-1 and VARC-2, and compared the mortality one year after the procedure. Kaplan-Meier curves showed numerically lower survival rates at one year by major VC with both definitions, but only VARC-2 had statistical significance: 79.3% vs. 60.7% with VARC-2 (p=0.014), and 78.9% vs. 70.5% with VARC-1 (p=0.20). Cox regression multivariable models showed major VC with VARC-2 definition to be an independent predictor of mortality (hazard ratio of 3.0, 95% confidence interval: 1.4-6.6, p=0.006), but not when it was substituted by the VARC-1 definition (p=0.15).
Conclusions: The VARC-2 definition of VC offers better predictive value of survival than the VARC-1 definition, supporting its efficacy as a standard definition.
Abbreviations
AS: aortic stenosis
CI: confidence interval
HR: hazard ratio
TAVI: transcatheter aortic valve implantation
VARC: Valve Academic Research Consortium
VC: vascular complications
Introduction
Since the introduction of transcatheter aortic valve implantation (TAVI), there has been substantial growth of this new technology. Although TAVI is established as the treatment of symptomatic aortic stenosis (AS) for high-risk or inoperable patients1,2, there remains controversy and many clinical investigations continue to emerge. The Valve Academic Research Consortium (VARC) consensus document was published in January 2011 with the goal of achieving consensus for: (i) selecting appropriate clinical endpoints reflecting device, procedure and patient-related effectiveness and safety, and (ii) standardising the definition for single and composite clinical endpoints, for TAVI clinical trials3. Since its publication, the clinical experience with this technology has matured and expanded, and certain definitions have become unsuitable or ambiguous. Thus, the selection and definition of clinical endpoints was revisited to make them more suitable to the present and future needs of clinical trials and the VARC-2 consensus was published in October 20124. However, the influence of this change in definition on clinical outcomes is unclear.
Vascular complications (VC) are important adverse events that are observed frequently during TAVI and associated with increased mortality5,6. The categorisation of this phenomenon is affected considerably by the definition changes: for example, Van Mieghem et al reported that the incidence of major VC in the same patient group varied from 5.2 to 15.9% depending on how they were defined7. In the updated VARC recommendations, there were two major VC definition changes: VC leading to an unplanned procedure NOT associated with a consecutive severe event was changed from a major to a minor complication, and the bleeding definition, in order to be a major VC, was changed from significant blood transfusion (≥4 U) to more than minor bleeding. These changes emphasise the importance of consequential events of VC, especially bleeding, and may reflect the clinical outcomes of TAVI more effectively. In this study, we sought to compare the predictive value for survival of major VC with VARC-1 and VARC-2 definitions.
Methods
PATIENT POPULATION
A single-centre series of patients consecutively undergoing TAVI from November 2007 to March 2012 was studied. TAVI was performed with the transfemoral or transapical approach using the Edwards SAPIEN (23 mm, 26 mm) or SAPIEN XT (23 mm, 26 mm, 29 mm) valve (Edwards Lifesciences, Irvine, CA, USA). A baseline invasive angiogram and/or CT angiogram of the iliofemoral artery was obtained. The transfemoral approach was considered as the primary access and the selection was determined based on the calibre of the artery, degree of calcium and severity of tortuosity. For the transfemoral approach, three types of the Edwards system introducer sheath were used: RetroFlex 3, NovaFlex and expandable sheath (eSheath). For the transapical approach, three types of the Edwards system introducer sheath were used: Ascendra, Ascendra 2 and Ascendra+.
PROCEDURE
TAVI was performed in the usual way. In transfemoral TAVI, the surgical approach was the primary method for arterial access and closure for device deployment from November 2007 to March 2011. Since March 2011, the complete percutaneous approach has been the primary method. The details of the surgical approach have been described elsewhere8. For the percutaneous approach, the two Perclose ProGlide® suture-mediated systems (Abbott Vascular Inc., Redwood City, CA, USA) were deployed for haemostasis. Transapical approach TAVI was performed in the standard way as described elsewhere9.
ENDPOINTS
We defined VC according to VARC-1 and VARC-2 definitions3,4. The major changes of the new definition were: VC leading to an unplanned procedure NOT associated with a consecutive severe event (death, major bleeding, visceral ischaemia or neurological impairment) was changed from a major to a minor complication, and the definition for bleeding being a major VC was changed from significant blood transfusion (≥4 U) to more than minor bleeding (overt bleeding either associated with a drop in the haemoglobin level of at least 3.0 g/dl or requiring transfusion of two or three units of whole blood/RBC, or causing hospitalisation or permanent injury, or requiring surgery).
STATISTICAL ANALYSIS
Data were collected retrospectively and all the analyses were performed with data from the as-treated population. Normality of distributions for continuous variables was tested using the Shapiro-Wilk test and data were analysed appropriately thereafter. Quantitative variables were expressed as mean±standard deviation for normally distributed variables and median (interquartile range) for non-normally distributed variables, and qualitative variables were expressed as number and percentage. Comparison of quantitative variables was performed with an independent samples t-test or a Mann-Whitney U test, depending on variable distribution. A chi-square test or a Fisher’s exact test was used to compare qualitative variables. If missing values existed, an available case analysis was performed. For assessment of the value of major VC based on VARC-1 and VARC-2 definitions, survival curves for time-to-event variables were constructed on the basis of all available follow-up data with the use of Kaplan-Meier estimates and were compared using the log-rank test. A multivariable analysis for one-year mortality in the TAVI patients was performed using a Cox regression model with forward stepwise analysis. The multivariable analysis incorporated all variables related to one-year mortality with a significance level of p≤0.1. A p-value ≤0.05 was considered statistically significant. All data were analysed with SPSS software, PASW statistics for Windows, Version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
PATIENT POPULATION
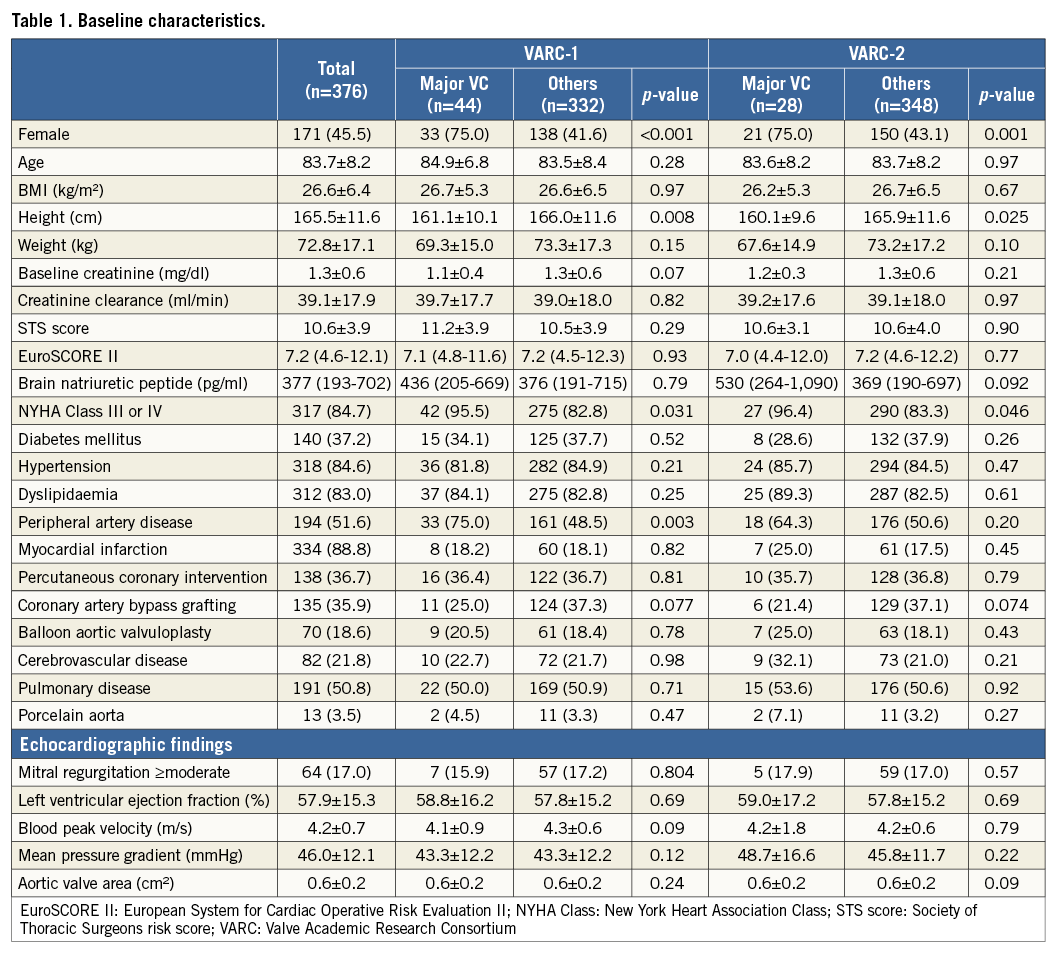
Data were analysed for 376 patients in a single centre. The transfemoral approach was performed in 322 cases (85.6%) and the transapical in 54 cases (14.4%). Based on the VARC-1 definition, 44 patients had major VC (11.7%). Based on the VARC-2 definition, only 28 patients had major VC (7.4%) (Table 1).
PATIENT CHARACTERISTICS WITH VARC-1 AND VARC-2
Baseline characteristics are described in Table 1. Overall, the mean age and the Society of Thoracic Surgeons score were 83.7±8.2 and 10.6±3.9, respectively. In both the VARC-1 and VARC-2 definitions, the major VC group showed higher female rates (VARC-1: 75.0% vs. 41.6%, p<0.001; VARC-2: 75.0% vs. 43.1%, p=0.001), lower height (VARC-1: 161.1±10.1 cm vs. 166.0±11.6 cm, p=0.002; VARC-2: 160.1±9.6 cm vs. 165.9±11.6 cm, p=0.008) and higher pre-TAVI New York Heart Association Class III or IV (VARC-1: 95.5% vs. 82.8%, p=0.031; VARC-2: 96.4% vs. 83.3%, p=0.046). In the VARC-1 definition, the VC group had a higher prevalence of peripheral artery disease: 75.0% vs. 48.5% (p=0.003); however, the significance disappeared using the VARC-2 definition: 64.3% vs. 50.6% (p=0.20).
Clinical characteristics are described in Table 2. In both the VARC-1 and VARC-2 definitions, the major VC group needed more RBC transfusion units (VARC-1: two [0.25-4] units vs. zero [0-1] units, p<0.001; VARC-2: three [2-6] units vs. zero [0-1] units, p<0.001) and had a greater sheath to iliofemoral artery ratio (VARC-1: 1.22±0.17 vs. 1.08±0.14, p<0.001; VARC-2: 1.22±0.16 vs. 1.09±0.15, p<0.001). In the VARC-1 definition, the major VC group revealed a higher transfemoral approach rate than the transapical approach (97.7% vs. 16.0%, p=0.015); however, the significance disappeared using the VARC-2 definition. On the other hand, the major VC group revealed a greater haemoglobin drop (3.1 [0.0-4.7] g/dl vs. 1.7 [0.2-2.6] g/dl, p=0.011) and longer hospital stay (five [3.25-9] days vs. three [2-6] days, p=0.041) using the VARC-2 definition; however, this significance disappeared in the VARC-1 definition.
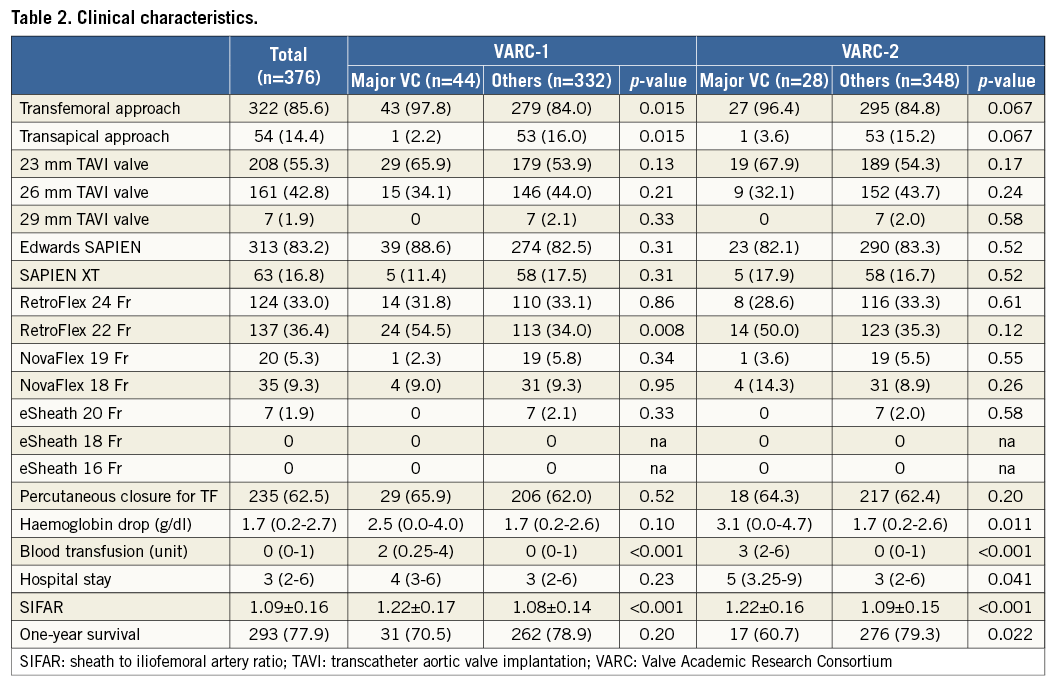
The definition changes from VARC-1 to VARC-2 caused some conversions in the VC population (Table 3). In the VARC-1 definition, the number of major VC due to unplanned procedures was 35 (9.3%), but in the VARC-2 definition, which requires a combination of an unplanned procedure and a consequent severe event, this was reduced to 17 (4.5%) (p<0.001). On the other hand, the number of major VC classified due to bleeding increased in the VARC-2 definition: eight (2.1%) vs. 26 (6.9%) (p<0.001).
MORTALITY WITH MAJOR VASCULAR COMPLICATIONS BASED ON VARC-1 AND VARC-2
Kaplan-Meier curves showed numerically lower survival rates at one year for the major VC group with both definitions, but only VARC-2 had statistical significance: 79.3% vs. 60.7% with VARC-2 (p=0.014), and 78.9% vs. 70.5% with VARC-1 (p=0.20). The hazard ratio (HR) calculated by univariate Cox regression models also showed significance only in the VARC-2 definition: 1.5 with VARC-1 (95% confidence interval [CI]: 0.8 to 2.7; p=0.20) and 2.2 with VARC-2 (95% CI: 1.2 to 4.10; p=0.017) (Figure 1).
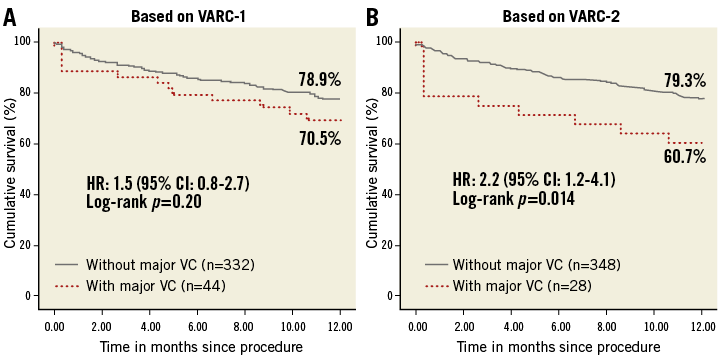
Figure 1. Kaplan-Meier curves showing cumulative survival rates up to one year for major vascular complications based on VARC-1 and VARC-2 definitions. CI: confidence interval; HR: hazard ratio; VARC: Valve Academic Research Consortium; VC: vascular complications
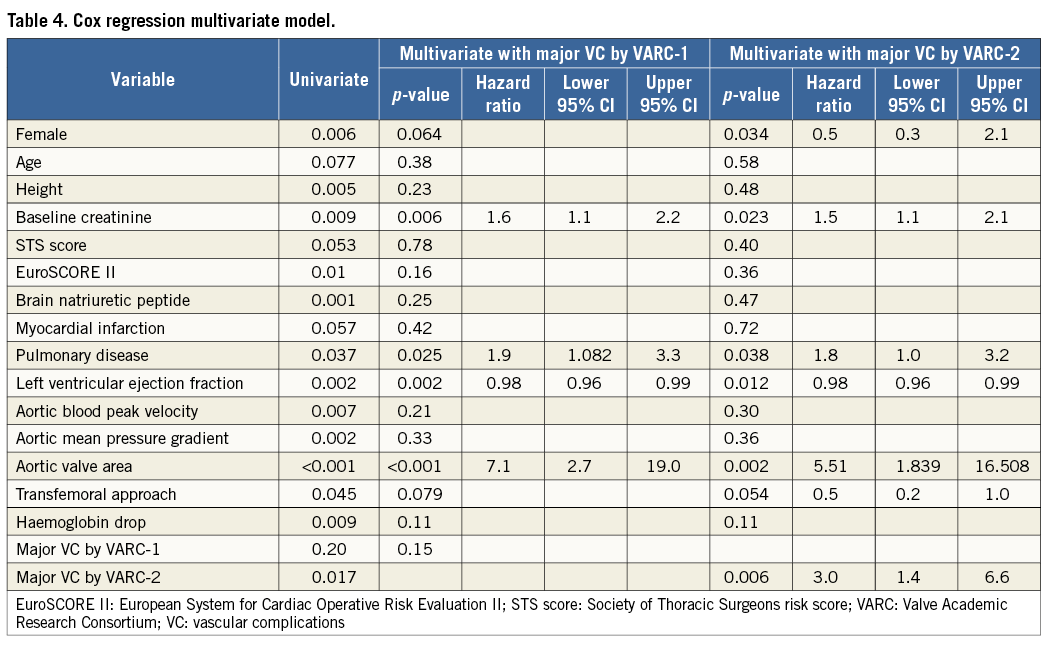
Furthermore, a Cox regression multivariable model was performed and showed major VC by the VARC-2 definition to be an independent predictor of one-year mortality with an HR of 3.0 (95% CI: 1.4 to 6.6; p=0.006); however, by the VARC-1 definition, major VC did not show a significant correlation to one-year mortality when it was substituted: HR of 2.1 (p=0.15) (Table 4).
Discussion
VC represent an important adverse event of TAVI which are frequently observed and can affect mortality5,6. However, the categorisation and grading severity of these complications is considerably affected by its definition.
Before the publication of the VARC-1 definition, VC were reported based on the categorisation of the site or investigator. Piazza et al reported vascular access-site complications in 12 out of 646 patients (1.9%). They reported separately on aortic root dissection or perforation (four cases; 0.6%) and on ventricular perforation (11 cases; 1.7%)10. Thomas et al defined major VC as limb-threatening ischaemia, vessel rupture requiring additional non-planned vascular surgery, or additional interventional treatment. It was seen in 49 patients (10.6%) out of 463 transfemoral approach cases and in 14 patients (2.4%) out of 575 transapical cases11. Different centres reported a wide range of VC rates which were changed depending on the definitions, and the value as a predictor for mortality was heterogeneous. Van Mieghem et al reported that the incidence of major VC in the same patient group varied from 5.2 to 15.9% depending on how they were defined7.
The VARC definition has provided uniformity in outcome reporting post TAVI3. In a meta-analysis by Généreux et al, based on the VARC-1 definition, the major VC rate was from 5% to 23.3% and the minor VC rate was from 5.6 to 28.3%12. Based on this definition, major VC have been identified as a predictor of mortality. Hayashida et al reported higher 30-day mortality with major VC: 22.7% vs. 7.6% (p=0.049)5. Généreux et al reported major VC as an independent predictor of one-year death with an HR of 2.31 (95% CI: 1.2 to 4.43; p=0.012)6. However, further clarification of the VARC definition was needed as a result of changes in the TAVI procedure and so the VARC-2 definition was introduced. The efficacy of this change was unknown. This study has revealed the value of VARC-2 as a predictor of one-year survival.
CONVERSION OF PATIENTS BASED ON VARC-1 AND VARC-2
To see the effect of definition change, we looked at the patient conversion brought about by the updated definition. Major VC according to the VARC-2 definition excluded patients who were treated well and had no consecutive events, and so the total number was decreased: 44 (11.7%) vs. 28 (7.4%), p<0.001.
VC are known to be the main cause of bleeding complications13. The relation of these two complications became stronger in the VARC-2 definition as the number of patients who are classified as having major VC based on bleeding complications increased significantly: eight (2.1%) vs. 26 (6.9%), p<0.001. Because of this change, only in the VARC-2 definition but not in the VARC-1 definition, patients with VC had a significant haemoglobin drop (3.1 [0.0-4.7] g/dl vs. 1.7 [0.2-2.6] g/dl, p=0.011).
MORTALITY BASED ON VARC-1 AND VARC-2
In our study, major VC based on VARC-1 did not show a significant relation with mortality, which was different from the reports of Hayashida and Généreux5,6. However, Stortecky et al also reported the outcome of VC as being comparable to patients without VC: the all-cause mortality rate at 30 days for patients with VC and without VC was 4% and 7%, respectively (p=0.60)14. They treated VC effectively with percutaneous management with a high success rate (21/23, 91%). The manuscript concluded that a percutaneous technique with rapid repair of vascular injury is desirable, especially among elderly high-risk patients undergoing TAVI, as it minimises blood loss and the risk of wound infections and also allows rapid mobilisation and earlier hospital discharge.
Our study had a relatively large number of patients and contained latter cases in the learning curve, with the majority of patients undergoing a percutaneous management. Experienced percutaneous management of VC might reduce the mortality in major VC by VARC-1.
Steinvil et al also examined the effect of VC definition on outcome following TAVI and had different results from the present study. They found that only VARC-1-defined major VC were significantly associated with increased mortality (HR 3.52, 95% CI: 1.5 to 8.4; p=0.005), whereas VARC-2-defined major VC were found to be only marginally significant (HR 1.9, 95% CI: 0.9 to 3.9; p=0.08)15. Heterogeneity of management of VC may explain this difference.
In this study, VC defined by only VARC-2 but not VARC-1 was found to be an independent predictor of one-year mortality. As previously mentioned, VARC-2 emphasises the consequent condition. Based on this finding, VC occurrence itself may not be strongly correlated to mortality but the condition caused by this complication is more meaningful, and the prompt and effective treatment of this phenomenon, which leads to a less consequent event, may result in a better outcome for the patient following TAVI. Overall, the change to the VARC-2 definition was reasonable in the current situation of wide use of a fully percutaneous procedure, and our study provided the evidence supporting this definition change.
Limitations
This was a single-centre study and the data were collected retrospectively. The inclusion period of this study was between 2007 and 2012. Since then, the procedure and technology have been further refined and therefore this population might not reflect the current population undergoing TAVI. During the study period only the balloon-expandable device was used in our institute although a self-expanding device was available elsewhere. The access site was also limited to only the transfemoral or the transapical whereas the subclavian-axillary artery or transaortic approaches were other options.
Conclusion
The VARC-2 definition for VC offers better stratification of survival than the VARC-1 definition, supporting its widespread use. Based on this finding, prompt and effective treatment of VC, which prevents consequent severe events, may bring better TAVI outcomes.
| Impact on daily practice The VARC-2 definition, which emphasises the consequent event of VC, reveals better survival prediction than the VARC-1 definition, supporting its widespread use in TAVI practice. Based on this finding, the condition caused by the complication is more meaningful than the VC occurrence itself. The prompt and effective treatment of this phenomenon, which leads to less consequential events, may result in a better outcome for the patient following TAVI. |
Conflict of interest statement
R. Makkar and H. Jilaihawi are consultants for Edwards Lifesciences. The other authors have no conflicts of interest to declare.

