Abstract
Aims: Transcatheter aortic valve replacement (TAVR) requires large calibre catheters and is therefore associated with increased vascular complications. The aim of this study was to illustrate the impact of the different definitions of major vascular complications on their incidence and to underscore the importance of uniform reporting.
Methods and results: We pooled dedicated databases of consecutive patients undergoing TAVR from two tertiary care facilities and looked for the incidence of major vascular complications using various previously reported definitions. The level of agreement (Kappa statistic) between the respective definitions and the Valve Academic Research Consortium (VARC) consensus definition of vascular complications was assessed. A total of 345 consecutive patients underwent transfemoral TAVR and were included in this analysis. A completely percutaneous access and closure technique was applied in 96% of cases. Arterial sheath size ranged between 18 and 24 Fr, the majority being 18 Fr (60%). Procedural success was reached in 94.5%. Depending on the definition used, major vascular complications occurred in 5.2-15.9% of patients. According to the VARC definitions, the rate of major and minor vascular complications was 9.0% and 9.6%, respectively. Major vascular complications according to VARC criteria demonstrated at least a substantial level of agreement with the SOURCE registry (κ 0.80) , the UK registry (κ 0.82) the Italian registry (κ 0.72) and “FRANCE” registry (κ 0.70) definitions, compared to a moderate level of agreement with the definitions used in the German registry (κ 0.47) and the 18 Fr Safety and Efficacy study (κ 0.42). Minor complications according to VARC demonstrated a moderate agreement only with vascular complications using the German registry definition (κ 0.54).
Conclusions: Non-uniformity in how vascular complications are defined precludes any reliable comparison between previously reported TAVR registries. The VARC consensus document offers standardised endpoint definitions and should be universally adopted to obtain better insights into global TAVR experience.
Abbreviations
AS: aortic valve stenosis
SAVR: surgical aortic valve replacement
TAVR: transcatheter aortic valve replacement
TF: transfemoral
VARC: Valve Academic Research Consortium
Introduction
Transcatheter aortic valve replacement (TAVR) has become an established treatment option for elderly patients with symptomatic severe aortic valve stenosis (AS) deemed inoperable or at high operative risk1-8. Many TAVR operators have adopted a “transfemoral (TF) first” approach, where transapical, direct aortic or transaxillary/subclavian strategies are alternative options in case of unfavourable anatomy of the femoro-iliac arterial tree. Although TF TAVR is a less invasive alternative to conventional surgical aortic valve replacement (SAVR) it still requires large calibre catheters. Vascular access-site complications are an inherent limitation of the current technology and impact on patient outcome, length of hospital stay, need for blood product transfusions and morbidity9-11. Multiple dedicated national and international TAVR registries have reported outcome data. Wide variations in the incidence of major vascular complications have emerged. The non-uniformity of the respective definitions used renders any attempt at interpretation and fair comparison throughout these registries futile. The Valve Academic Research Consortium (VARC) is a collaborative academic initiative to address this non-uniformity in reporting by generating standardised consensus definitions for important clinical TAVR endpoints, ultimately to improve the quality of clinical research and facilitate relevant comparison between reports12,13.
The aim of the present study was to illustrate the impact of different definitions on the incidence of vascular complications and assess the level of agreement between the respective definitions.
Methods
PATIENT POPULATION
Between November 2005 and August 2011, 345 consecutive patients from two tertiary care facilities underwent TAVR via TF approach and were included in this study. Patient operative risk status and eligibility for the TAVR procedure were determined by a multidisciplinary Heart Team (consisting of at least one interventional cardiologist and one cardiothoracic surgeon) consensus, based on calculated risk scores (Society of Thoracic Surgeons [STS], logistic EuroSCORE), the interpretation of other risk variables not captured by these established risk models (frailty, porcelain aorta, previous mediastinal radiation, chest wall deformity, etc.), and clinical judgement. All patients consented to the TAVR procedure and use of related data for research and publication purposes in accordance with Institutional Review Board approval.
Both Edwards SAPIEN™ (Edwards Lifesciences Inc., Irvine, CA, USA) and Medtronic CoreValve® (Medtronic Inc., Minneapolis, MN, USA) device platforms were used. Baseline patient characteristics, procedural details and clinical outcome data were prospectively collected. After the VARC consensus document was made public, the proposed endpoint definitions were adopted and the respective local databases were modified accordingly. All data were then merged into a global dataset for retrospective analysis.
ENDPOINT DEFINITIONS
Vascular complications were reported according to both existing definitions used in various multicentre registries and the VARC consensus definitions (Table 1 and Table 2)12,13. Procedural success was defined as successful vascular access, delivery and deployment of the device and successful retrieval of the delivery system with good performance of the prosthetic heart valve (aortic valve area >1.2 cm2 and mean aortic valve gradient <20 mmHg or peak velocity <3 m/s, and aortic regurgitation [AR] <2) and only one valve implanted in the proper anatomical location. Three independent interventional cardiologists knowledgeable of TAVR procedures adjudicated all events retrospectively. Conflicts were resolved by consensus.
STATISTICAL ANALYSIS
Categorical variables are presented as frequencies and percentages; continuous variables are presented as mean (±SD) in case of a normal distribution or medians (interquartile range [IQR]) in case of a skewed distribution. Normality of the distributions was assessed using the Shapiro-Wilk test. Definitions for vascular complication as used in reported registries were compared to the VARC major and minor vascular complication definitions. The agreement between definitions was examined by performing weighted Kappa calculations where a Kappa value of <0.20, 0.21-0.40, 0.41-0.60, 0.61-0.80, and >0.80-1.0 is interpreted as showing slight, fair, moderate, substantial and almost perfect agreement, respectively (12). The predictive value of vascular complications according to the various different definitions on 30-day mortality was assessed by univariable logistic regression analysis. A two-sided alpha level of 0.05 was considered to be statistically significant. All analyses were performed with SPSS software 17.0 (SPSS Inc, Chicago, IL, USA).
Results
Baseline patient characteristics are depicted in Table 3. A total of 345 patients with a mean age of 82.3±8.0 years were included; 52% were male. Median STS score was 5.0 (IQR 2.95-7.05). Table 4 illustrates procedural characteristics. The majority of patients (95.7%) underwent transfemoral TAVR using a completely percutaneous approach, including arteriotomy closure with a closure device. Arterial sheath size varied from 18 to 24 Fr; 60% of cases involved using 18 Fr arterial sheaths. Procedural success was 94.5%.
CLINICAL OUTCOME
All-cause 30-day mortality was 6.4% and the major stroke rate was 4.6% (Table 5). According to the VARC definitions there were 9.0% (31/345 patients) of major and 9.6% (33/345 patients) of minor vascular complications. Closure device failure occurred in 3.8% (13/345) of all patients, accounting for 41.9% and 39.4% of all major and minor vascular complications, respectively.
LEVEL OF AGREEMENT BETWEEN VASCULAR COMPLICATIONS DEFINITIONS
Figure 1 compares the frequency of vascular complications according to the various registry definitions. The frequency of vascular complications would be highest with the German registry definition and lowest with the UK registry definition. The level of agreement (Kappa statistic) was assessed between the major and minor VARC definitions and the other reported definitions. Major vascular complications according to VARC demonstrated excellent agreement with the SOURCE registry (κ 0.80) and the UK registry (κ 0.82) definition, substantial agreement with the Italian registry (κ 0.72) and “FRANCE” registry (κ 0.70) and only moderate agreement with the definitions used in the German registry (κ 0.47) and the 18 Fr Safety and Efficacy study (κ 0.42) (Figure 2A). Minor complications according to VARC demonstrated moderate agreement with vascular complications using the German registry definition (κ 0.54) (Figure 2B).
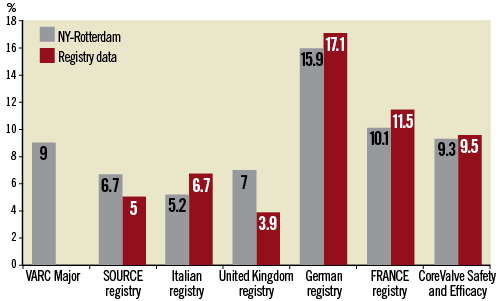
Figure 1. Incidence of major vascular complications (%) according to different definitions.

Figure 2. A) Level of agreement between VARC definition of major vascular complications and registry definitions; B) Level of agreement between VARC definition of minor vascular complications and registry definitions
Discussion
This pooled analysis containing a total of 345 high-risk AS patients undergoing TF TAVR looked at the frequency of vascular complications using and comparing various reported definitions. The apparent heterogeneity in these definitions illustrates how difficult it may be to compare outcome data from TAVR registries in the absence of uniformity in endpoint definitions and underscores the importance of the VARC concept.
In this series, the rate of major vascular complications varied from 5.2 to 15.9% depending on the definition used.
The VARC definition of major vascular complications showed strong agreement with the definition used in both the SOURCE and UK registries7,8. The UK registry definition is more generic and leaves room for interpretation, which can influence event adjudication (e.g., what injury is considered major?) and introduce bias. Conversely, the German registry definition lacks descriptive value3. VARC proposes a more detailed and TAVR-tailored definition of vascular complications.
The frequency of major vascular complications was 9% using the VARC consensus definition. Arteriotomy closure devices are widely used in TF TAVR. In this series, closure device failure led to major vascular complications in 3% corresponding to about 40% of the total burden of major vascular complications. VARC specifically chose to qualify closure device failure as a minor complication unless it was associated with considerable morbidity (end-organ damage, >4 units red blood cell [RBC] transfusion) or mortality. The use and success of closure devices merits continuous assessment as more advanced arteriotomy closure technologies may have higher success rates and may substantially reduce vascular complications.
Other groups have reported VARC major vascular complications rates of between 5 and 23% (13). Apart from experience level, the retrospective nature of these reports may partly explain this wide variation. Evidently prospectively collected data using the VARC consensus definitions may further improve data comparability.
Therefore the global adoption of these VARC endpoint definitions by centres and registries should be encouraged.
Limitations
This study contains the inherent limitations of all retrospective analyses. Vascular complications were not adjudicated by an independent clinical event committee, which can introduce potential reporting bias. The definitions used in previous registries leave room for interpretation and therefore the comparison between our study results and those of the respective registries can only be directive at best.
Nevertheless, the main purpose of this study was to illustrate the impact of using non-uniform endpoint definitions.
Conclusion
This study underscores the importance of uniformity in how clinical endpoints are defined and should help persuade centres and trial initiatives to adopt the VARC consensus definitions when reporting on TAVR outcome and clinical endpoints. Undoubtedly, TAVR reports using uniform consensus definitions will provide better insights into the global TAVR experience and may help national regulatory bodies put the TAVR technology into proper perspective and perhaps guide appropriate reimbursement policies in the near future.
Conflict of interest statement
P. Généreux has received speaker honoraria from Edwards Lifesciences. S. Kodali and M. Williams have received consulting fees from Edwards Lifesciences. P.P. de Jaegere is a proctor for Medtronic CoreValve. M.B. Leon is a non-paid member of the Scientific Advisory Board of Edwards Lifesciences and Medtronic Vascular.The other authors have no conflicts of interest to declare.

