Abstract
Aims: Transcatheter aortic valve implantation (TAVI) requires large bore catheters. Access site complications, therefore, can be a concern. The aim of this study is to present the 30-day incidence of major and minor vascular complications in patients treated with the third generation 18 Fr Medtronic CoreValve System®.
Methods and results: We prospectively evaluated the vascular complications occurring in all patients treated with the 18 Fr Medtronic CoreValve System® between October 2006 and October 2009 in the Thoraxcenter using various proposed definitions. Ninety-nine consecutive patients were treated with TAVI using the 18 Fr Medtronic CoreValve System®. Vascular events were encountered in 13 patients (13%), seven of these cases (54%) were related to incomplete arteriotomy closure with the Prostar device which is the default access closure technique in our centre. Depending on how major vascular complications were defined, the incidence varied from 4 to 13%. Blood transfusions in combination with surgical or percutaneous intervention were required in eight cases.
Conclusions: Transcatheter aortic valve implantation with the 18 Fr Medtronic CoreValve System® has a 4 to 13% vascular complications’ rate. More than half of the vascular events were due to incomplete Prostar arteriotomy closure, despite its use by experienced operators. Current percutaneous closure devices for these large arteriotomies seems suboptimal. Uniformity in how to define TAVI related vascular complications is needed.
Introduction
The Medtronic CoreValve System® (Medtronic, Minneapolis, MN, USA) and the Edwards SAPIEN™ valve (Edwards Lifesciences, Irvine, California, USA) are the only two device platforms with CE Mark approval for transcatheter aortic valve implantation (TAVI)1,2. Since the presentation of the First-In-Man TAVI procedure in 2002, several device iterations have led to design changes creating lower profile platforms3. Currently, the Edwards and CoreValve delivery catheter systems are available in 22 Fr and 24 Fr, and 18 Fr size, respectively. Lower profile devices could be associated with less vascular complications and improved patient outcomes. The 18 Fr CoreValve Expanded Evaluation Registry reported access site bleeding in 2.6%, major bleeding in 6% and aortic dissection in 0.7%, whereas in the Edwards Partner EU trial the vascular complications rate (including bleeding) was 26%4,5. Non-uniformity, however, in the definitions used to report complications can make the comparison of data presented by various groups and with different device platforms a challenging endeavour6. The aim of this study is to present the 30-day incidence of major and minor vascular complications in patients treated with the third generation 18 Fr Medtronic CoreValve System®.
Methods
We prospectively enrolled 99 consecutive patients treated with the third generation 18 Fr Medtronic CoreValve System® between October 2006 and October 2009 at the Thoraxcentre, Rotterdam, The Netherlands. Each patient was deemed high risk or surgically inoperable by the Heart Team (specifically, an interventional cardiologist and cardiothoracic surgeon).
To select the appropriate vascular access site, patients underwent peripheral angiography and/or multislice computed tomography (MSCT) to assess vessel size, degree of calcification, tortuosity and atherosclerotic burden of the aortic-ilio-femoral tree. (Figure 1)
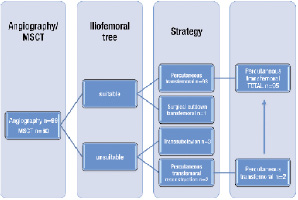
Figure 1. Access strategy flow chart.
The transfemoral approach was used as the default access site. The minimum acceptable vessel size was 6 mm. A borderline vessel size, with only mild calcification or atherosclerotic burden, was deemed flexible enough to accommodate the 18 Fr sheath. When the ilio-femoral vessels were inaccessible (vessel size, calcification, tortuosity, atherosclerosis), the subclavian approach was selected. Percutaneous ilio-femoral reconstruction was also an option in case of significant obstructive atherosclerotic disease7 (Figure 2).
Surgical cut-down is standard in the subclavian access strategy.
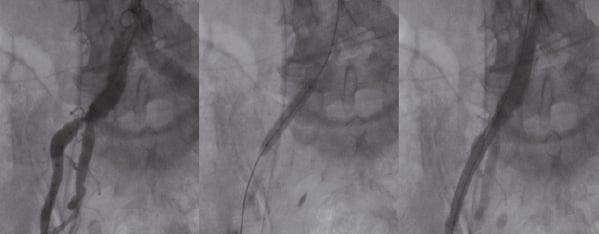
Figure 2. Femoral reconstruction before TAVI. Left panel: Severe atherosclerotic disease in the right common iliac artery. Middle panel: stenting of the right common iliac artery. Right panel: Right common iliac artery after stenting, showing adequate vessel size to accommodate the 18 Fr device platform.
Definition of endpoints
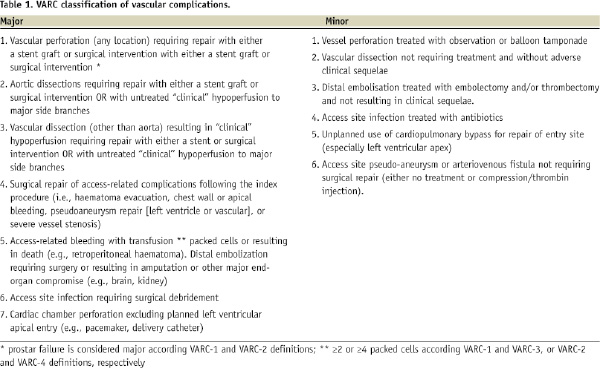
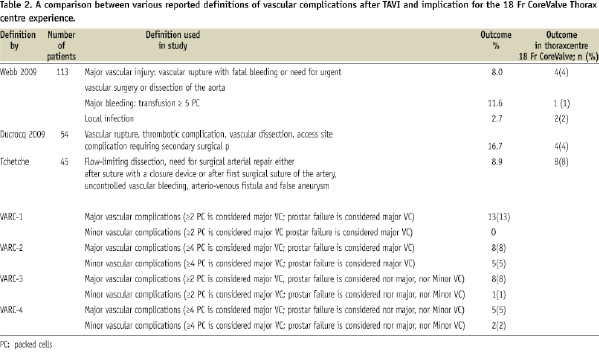
There is no uniformity in how to define major vascular complications. Therefore, we used several definitions encountered in the literature. Also, the Valvular Academic Research Consortium (VARC), a collaboration between academic research organizations in the United States and Europe is in the process of preparing a consensus document on TAVI related endpoint definitions. For vascular complications, the relative importance of blood transfusions (what amount of transfused packed cells is clinically relevant?) and whether surgical correction of a failed Prostar arteriotomy closure should be accounted for, is still a matter of debate. We therefore presented four different preliminary VARC definitions (Table 1). A summary of various proposed definitions with the subsequent impact on the prevalence of vascular complications in our experience is presented in Table 2.
Statistical analysis
Continuous variables are expressed as mean±standard deviation and categorical variables as percentages. A p value ≤0.05 is considered statistically significant.
Results
Between October 2006 and October 2009 we prospectively enrolled 99 consecutive patients treated with the 18 Fr Medtronic CoreValve System®. Patient characteristics are presented in Table 3. The vascular access strategy is shown in Figure 1. The transfemoral strategy was selected in 96 patients with upfront percutaneous femoral reconstruction in two (Figure 2) and surgical cut-down in one. Three patients underwent the subclavian approach.
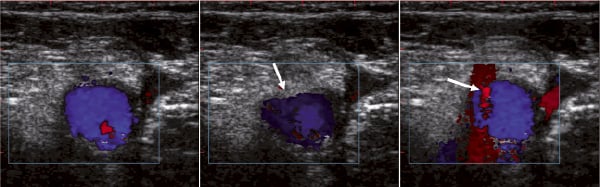
Figure 3. Ultrasound guided femoral artery puncture. Left: baseline femoral artery (Blue color Doppler). Middle: tenting of the artery by the approaching needle (arrow). Right: needle entry in the anterior vessel wall with typical red color doppler sign (arrow).
A description of patient-level vascular complications according to the different proposed VARC definitions is presented in Table 4. Vascular events with the 18 Fr Medtronic CoreValve System® were encountered in 13 patients (13%).
Incomplete arteriotomy closure with the Prostar XL device led to overt bleeding in seven cases: two were treated with percutaneous stenting, five required a surgical repair. In two patients, this vascular complication triggered a train of events resulting in sepsis and subsequent death in one, and critical leg ischaemia requiring vascular surgical intervention in another.
Four localised groin haematomas were treated with blood transfusions only. Occult bleeding with retroperitoneal haemorrhage was found in two patients, one required surgical exploration (Figure 4).
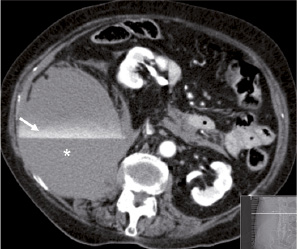
Figure 4. MSCT scan showing a large retroperitoneal haematoma (*) with active bleeding component (contrast line marked by the arrow) lifting the right kidney (+) anteriorly and medially.
Major access site complications were not associated with any statistically significant difference in length of stay in hospital (LOS) or mortality compared to patients with no access site complications, although the numbers were too small to draw firm conclusions.
Discussion
The aim of our study was to focus on vascular complications related to TAVI using the 18 Fr Medtronic CoreValve System®. Vascular events were observed in 13 patients. Seven of the 13 vascular events (54%) were the result of Prostar failure to obtain adequate haemostasis. Surgical exploration was required in six cases, percutaneous covered stent implantation in two. Eight of these events required blood transfusions.
Depending on how major vascular complications were defined, the prevalence in our centre varied from 4 to 13%. In their respective cohorts of patients treated with the Edwards SAPIEN™ valve, Webb et al found a vascular injury rate of 8% in 113 patients9, whereas Ducrocq et al had a vascular complication rate of 16.7% in 54 patients10. The Canadian multicentre program of compassionate clinical use of the Edwards platform in 168 TAVI patients presented a 13% incidence of major access site complications associated with a 25% mortality rate11. Tchetche et al reported a vascular complication rate of 8.9% in a mixed cohort of 45 patients treated with the Edwards or Medtronic platform12. A single-centre registry of 153 transfemoral TAVI procedures predominantly using the CoreValve System® reported a femoral vessel complication rate of 16%13. In a large multicentre registry with the 18 Fr CoreValve System® including 646 TAVI patients, Piazza et al found a vascular access site complication rate defined as dissection or vascular tear of 1.9%14. Of note, the identification of surgical repair after Prostar failure, and the relative impact of blood transfusions contributing to the definition of vascular complications, differs in the various proposed definitions.
Preventive strategies can be implemented in every facet of the vascular access procedure. Judicious patient selection, with pre-procedural imaging to assess aorto-ilio-femoral calibre, calcification, atherosclerosis and tortuosity is crucial15,16. In our department, MSCT scans of the aorto-ilio-femoral tree is becoming standard practice in addition to peripheral angiography and ultrasound examination. Alternative access sites can be considered: trans-subclavian, trans-axillary, trans-apical, trans-aortic or retroperitoneal strategies have all been described, but usually come with increased invasiveness and periprocedural morbidity18-21.
The technique of echo guided arterial and venous femoral access ensures correct entry in the common femoral artery in a disease free area, avoiding superficial femoral artery and posterior/lateral arterial wall puncture, both notorious predictors of occult and overt bleeding, as well as other complications like arterio-venous fistula formation8,17.
Other possible alternative devices to obtain uncomplicated arterial access are the micro-puncture kit (Cook, Inc., Bloomington, IN, USA), which includes a 21 gauge needle for initial access and the Smartneedle (Peripheral Systems Group, Mountain View, CA, USA), consisting of a miniaturised 20 gauge Doppler transducer inserted within an 18 gauge cannulation needle for location and cannulation of peripheral vessels.
Furthermore, fluoroscopic guidance while advancing the large bore catheters allows the operator to appreciate complicated vessel features and control guidewire movement. In selected cases with significant atherosclerotic burden but acceptable vessel size, percutaneous vessel reconstruction before TAVI might render the femoral access once again feasible7. Half of the vascular events in our study were related to incomplete arteriotomy closure by the Prostar XL system. Patient selection (e.g., excessive femoral artery calcification and obesity, would favour surgical closure) and the operator’s learning curve in deploying the Prostar device can contribute to these events. The Prostar device was originally designed for a suture-based 10 Fr arteriotomy closure. However, it is commonly used for closing arterial access sites up to 18 Fr. Alternative percutaneous closure techniques for these large arteriotomies such as closure with two Prostar devices or two or three Perclose devices (Abbott Vascular Devices, Redwood City, CA, USA) have been described, although there is always room for continuous technical improvement in this domain22,23.
Downsizing of the device profile can also improve procedural outcomes, and one can anticipate that smaller systems will further reduce vascular complications. In this respect, Edwards developed the Novaflex delivery system, reducing the delivery catheter profile from 24 Fr and 22 Fr to 19 Fr and 18 Fr for the large and small valve sizes, respectively.
Apart from the evident technical revolution, the impact of the learning curve underscores the importance of the operator’s experience in handling these specific devices and reaching a high rate of procedural success9,24.
Limitations
The limitations of the present study are evident: we present a single-centre analysis of a relatively small cohort of 99 patients treated with the CoreValve System®. Furthermore there is no uniformity in how to define access site complications in the literature. We report vascular complications using different definitions to allow a better comparison with other presented data.
Conclusion
TAVI with the 18 Fr Medtronic CoreValve System® appears to have an acceptable track record as far as vascular safety issues is concerned. Depending on which definition we used, major vascular complications were encountered in 4 to 13% of our study population. Prostar failure to close the arteriotomy and achieve complete haemostasis was the predominant cause. With further device iterations and technical refinements, the frequency of vascular complications will likely decrease.

