Abstract
Aims: The aim of this study was to compare outcomes with the use of two haemostasis strategies after transfemoral transcatheter aortic valve implantation (TAVI) – one Prostar® vs. two ProGlide® devices (Abbott Vascular Inc., Santa Clara, CA, USA).
Methods and results: This was a retrospective study enrolling consecutive patients undergoing fully percutaneous transfemoral TAVI in our centre (Ferrarotto Hospital, Catania, Italy) from January 2012 to October 2014. All patients were dichotomised according to the vascular closure device (VCD) used for common femoral artery haemostasis (Prostar vs. ProGlide). All outcomes were defined according to VARC-2 criteria. The study population encompassed a total of 278 patients. Of these, 153 (55.1%) underwent TAVI using the Prostar, and 125 (44.9%) using two ProGlide devices. Vascular complications occurred in 48 patients (17.3%), being more frequent in the ProGlide group (11.8% vs. 24.0%, p=0.007). Patients who had TAVI using the ProGlide were also more likely to have a higher rate of percutaneous closure device failure (4.6% vs. 12.8%, p=0.013). Percutaneous peripheral intervention was performed in 13.7% and 28.0% of Prostar and ProGlide cases, respectively (p=0.003).
Conclusions: Patients undergoing transfemoral TAVI had significantly lower rates of vascular complications and percutaneous closure device failures when the Prostar was used compared with two ProGlide devices.
Abbreviations
CI: confidence interval
MDCT: multi-detector computed tomography
OR: odds ratio
TAVI: transcatheter aortic valve implantation
VARC: Valve Academic Research Consortium
VCD: vascular closure device
Introduction
In the endovascular procedure setting, vascular closure devices (VCD) have emerged as an alternative to mechanical compression in order to achieve vascular haemostasis after puncture of the femoral artery1. The development of the transfemoral transcatheter aortic valve implantation (TAVI) technique in clinical practice has generated the need for VCD capable of accomplishing effective haemostasis after large diameter arteriotomies (up to 24 Fr, recently down to 14 Fr)2. Initially, open surgical access was routinely used to introduce large sheaths and catheters3,4. Subsequently, percutaneous techniques have emerged as the new standard, resulting in a less invasive, fully percutaneous procedure5. Because there are no available percutaneous devices specifically intended for large vessel closure, preclosure with either the 10 Fr Prostar XL® or two 6 Fr ProGlide® devices (Abbott Vascular Inc., Santa Clara, CA, USA) are commonly used for this purpose5,6. Both of them have been shown to be effective7,8. However, no studies comparing these two closure approaches have been published so far. The aim of this analysis was therefore to compare the acute outcomes of these two strategies for percutaneous vascular closure of large arteriotomies in the setting of transfemoral TAVI.
Methods
PATIENT POPULATION
In this retrospective analysis the current study population encompassed a total of 278 consecutive patients who underwent transfemoral TAVI from January 2012 to October 2014. Of these, 153 (55.1%) underwent TAVI using the Prostar XL (Prostar group), and 125 (44.9%) using two ProGlide devices (ProGlide group). Two hundred and fifty patients treated with transfemoral TAVI before 2012 were excluded from this analysis in order to reduce the impact of the learning curve on the primary outcomes. In this particular population, both VCD were used in more than 150 cases. During the study period no cases of planned surgical cutdown were performed. All patients gave written informed consent for TAVI procedure.
SHEATHS AND ARTERIAL CLOSURE
The following sheaths were used - 18 Fr Check-Flo® (Cook Medical, Bloomington, IN, USA) for the CoreValve® (Medtronic, Minneapolis, MN, USA), and the 18 Fr Ultimum™ (St. Jude Medical, St. Paul, MN, USA) sheath for the Portico™ (St. Jude Medical) valve; 14/16/18/20 Fr expandable eSheaths (Edwards Lifesciences, Irvine, CA, USA) for the SAPIEN XT and SAPIEN 3 valves (Edwards Lifesciences). The design and mechanism of the Prostar XL and Perclose ProGlide devices have been described previously6. Prophylactic placement of a crossover wire (V-18™; Boston Scientific, Marlborough, MA, USA) from the contralateral femoral artery was performed in all patients. During the procedure, 100 IU/kg of unfractionated heparin was administered to achieve an activated clotting time of 250-300 seconds. All patients received aspirin 100 mg the day before the procedure. Clopidogrel was not given to any patient.
VASCULAR ACCESS EVALUATION
Before TAVI, peripheral access evaluation was accomplished with angiography of the descending aorta, iliac and femoral arteries, measuring the minimal lumen diameter to the level of the femoral head, and with multi-detector computed tomography (MDCT) by measuring the minimal lumen diameter using a centreline technique. Fluoroscopic calcification was graded as none, mild (some calcification), moderate (the course of the artery can be seen without injection of contrast dye), or severe (heavily calcified iliofemoral arteries). MDCT calcification was graded similarly7,8.
After large sheath removal, the puncture site was checked through selective injection of contrast dye in the external iliac artery.
TREATMENT OF VASCULAR COMPLICATIONS
Management of vascular complications was left to the operators’ discretion. Usually, iliofemoral dissections or stenoses were treated with conventional balloon angioplasty or, if necessary, self-expandable non-covered stents. Iliofemoral perforations causing residual bleeding, insufficiently managed with 15-20 minutes manual compression (first step) or balloon angioplasty (second step), were treated with covered stents or emergency surgery if percutaneous therapy failed or was not achievable. Protamine was utilised occasionally in cases of persistent bleeding.
STATISTICAL ANALYSIS AND DEFINITIONS
Descriptive statistics are reported as mean±standard deviation. Categorical variables were compared using the χ2 test and Fisher’s exact test. Normality of distribution was tested by means of the Kolmogorov-Smirnov test. Continuous Gaussian variables were compared by means of a Student’s t-test for independent samples, while skewed distributions were compared using the Mann-Whitney non-parametric test. Odds ratio (OR) and 95% confidence interval (CI) were calculated for multivariate predictors of any vascular complications and percutaneous closure device failure. Variables were included if they were found significant at 0.20 at univariate analysis or if considered clinically relevant. A two-sided p-value of less than 0.05 was considered to be of statistical significance. All data were processed using the Statistical Package for Social Sciences, Version 20 (IBM Corp., Armonk, NY, USA).
Clinical endpoints and definitions were used in accordance with the Valve Academic Research Consortium (VARC)-2 standardised endpoint definitions for TAVI9.
Results
PATIENT POPULATION
Baseline characteristics are listed in Table 1. Patients who had TAVI with the Prostar presented more frequently with permanent AF compared with those undergoing TAVI with the ProGlide (15.7% vs. 7.3%, p=0.031); otherwise, comorbidities across the study groups were equally distributed. Angiographically, there were no differences between these two groups in terms of minimal femoral artery diameter (7.4±1.2 vs. 7.3±1.3 mm, p=0.476), sheath external diameter/minimal femoral artery diameter ratio (0.99±0.18 vs. 1.01±0.20, p=0.232), and moderate/severe common femoral artery calcification (15.0% vs. 14.4%, p=0.643). Similar findings were reported when the femoral artery diameter was measured using MDCT (Table 1).
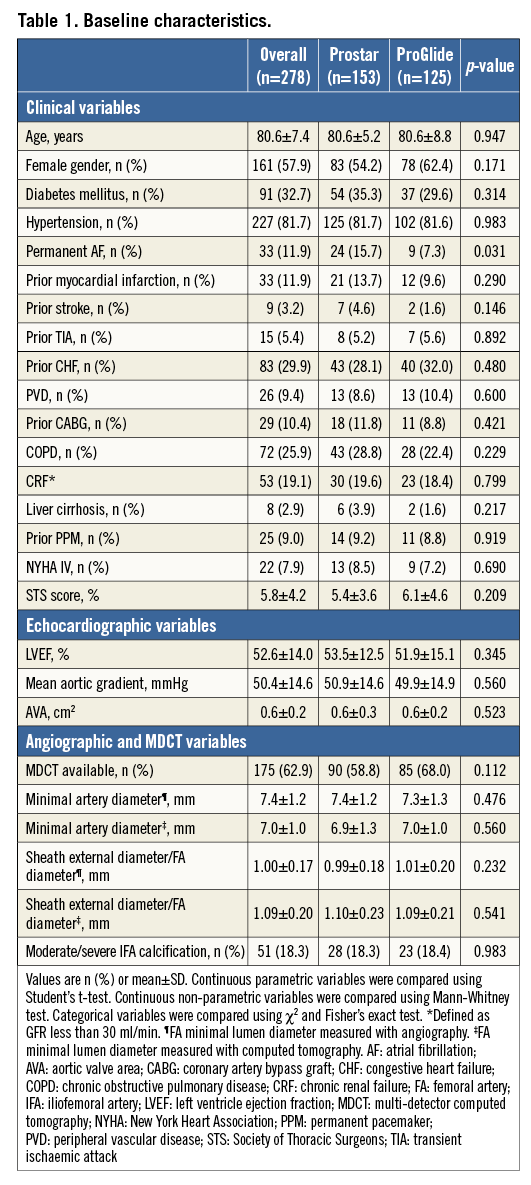
PROCEDURAL OUTCOMES
The main procedural variables are reported in Table 2. The device success rate was 88.1%, with no differences between groups. The vast majority of the procedures (95.6%) were accomplished by using the SAPIEN XT and the CoreValve prostheses, with no differences between the two groups. As a consequence, the sheath diameters used were also similar (Table 2). After large sheath removal, the deployment of one or more additional ProGlide devices to obtain proper haemostasis was required in 5.3% and 9.7% of cases in the Prostar and ProGlide groups, respectively (p=NS). Full details of vascular injury types across the study population are reported in Figure 1. All cases of dissection, residual stenosis and femoral occlusion were clinically silent (100%): they were diagnosed through selective angiography from the contralateral artery after sheath removal. On the other hand, 85.7% of residual bleeding and combined bleeding and dissections were clinically evident. Percutaneous peripheral intervention was performed in 55 patients, being more frequent across the ProGlide group (13.7% vs. 28.0%, p=0.003). Covered and non-covered stent implantations on the common femoral artery were required in 2.6% and 3.3% of patients in the Prostar group, and 5.6% and 9.6% of patients in the ProGlide group, respectively, whereas percutaneous peripheral intervention with balloon only was carried out in 7.9% and 19.2% of cases, respectively (Figure 2). Overall, unsuccessful haemostasis with balloon only, which required implantation of a covered stent, was reported in 2.6% and 3.2% of cases, respectively (p=NS). On the other hand, no differences were observed between groups in terms of unplanned vascular surgery (1.3% vs. 4.0%, p=0.149) (Figure 2).
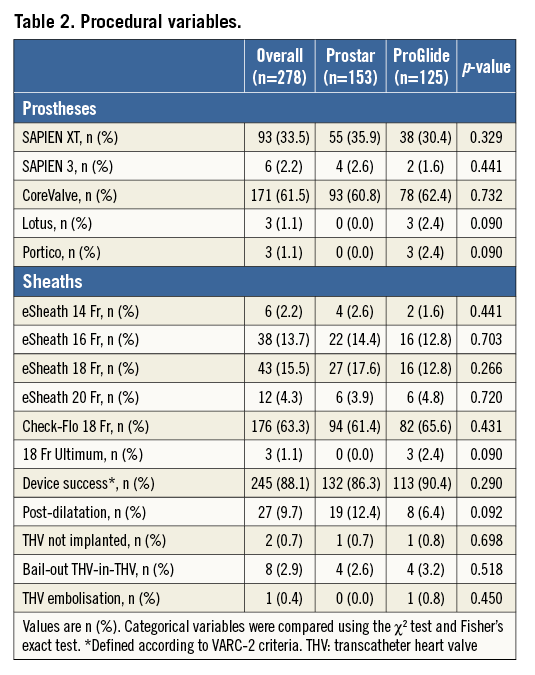
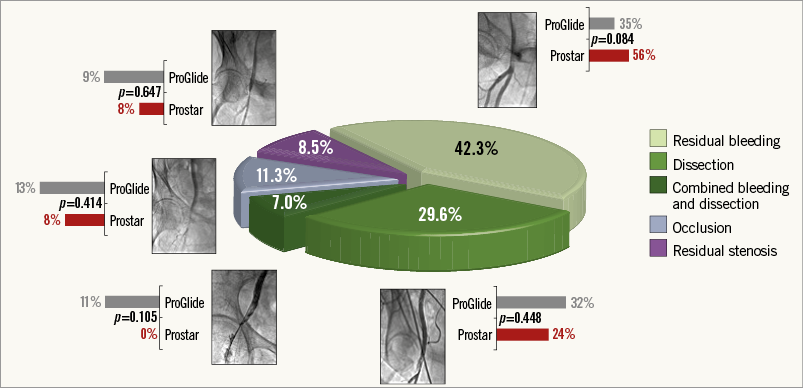
Figure 1. Vascular complications. Vascular injury types across the study population. The grey and the red bars indicate the rates of each complication in the ProGlide and the Prostar groups, respectively.
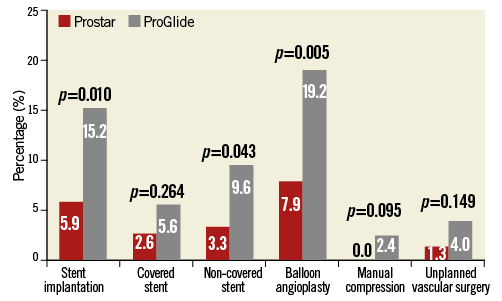
Figure 2. Vascular interventions. Difference in vascular intervention rates between patients having transfemoral TAVI with the Prostar (red bar) and the ProGlide (grey bar) devices.
IN-HOSPITAL OUTCOMES
Incidences of vascular complications over time in both groups are depicted in Figure 3. Overall, VARC-2 defined vascular complications occurred in 48 patients (17.3%), being more frequent in the ProGlide group (11.4% vs. 24.0%, p=0.007). Patients who had TAVI using the ProGlide were also more likely to have a higher rate of VARC-2 defined percutaneous closure device failure (4.6% vs. 12.8%, p=0.013) (Figure 4).
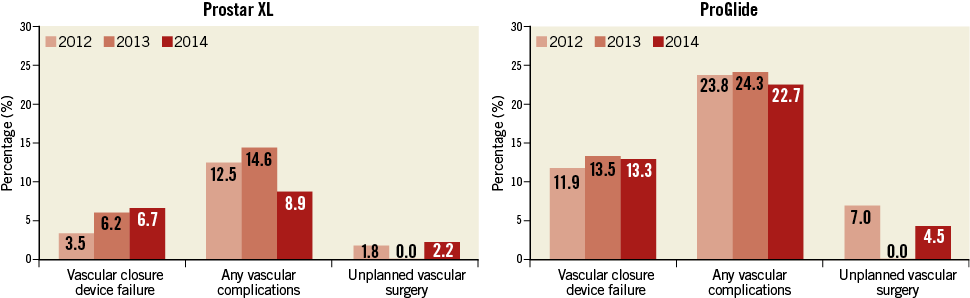
Figure 3. Vascular complications over time. Vascular closure device failure, vascular complications and unplanned vascular surgery incidences during the study period in the Prostar XL (left panel) and ProGlide (right panel) groups.
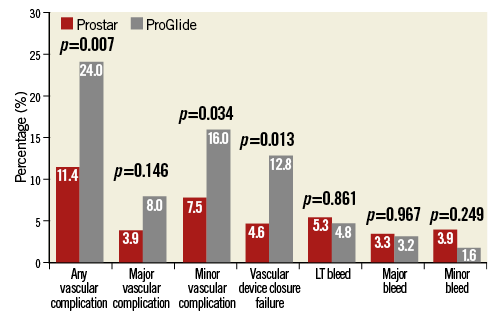
Figure 4. Vascular complications and bleeding. Difference in rates of VARC-2 defined vascular complications and bleeding between patients having transfemoral TAVI with the Prostar (red bar) and the ProGlide (grey bar) devices. LT: life-threatening
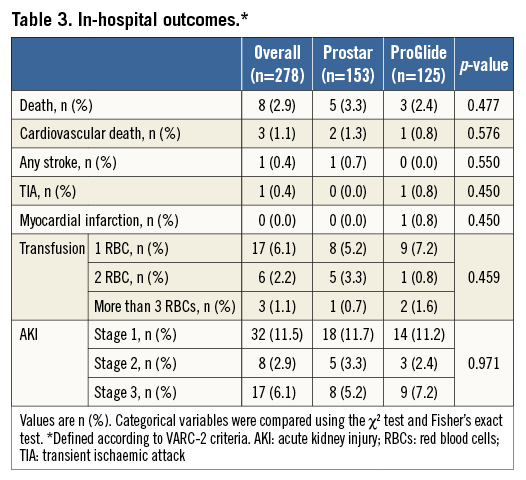
In-hospital clinical outcomes are summarised in Table 3. There were no differences between groups in terms of mortality (3.3% vs. 2.4%, p=0.477), stroke/TIA (0.7% vs. 0.8%, p=0.698), acute kidney injury 2 or 3 (8.5% vs. 9.6%, p=0.971), and any bleeding (12.5% vs. 9.6%, p=0.446).
MULTIVARIATE ANALYSIS
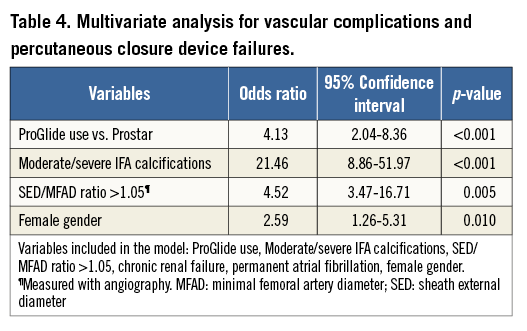
At multivariable analysis, the use of the ProGlide (adjusted OR 4.13, 95% CI: 2.04-8.36; p<0.001), moderate/severe iliofemoral artery calcifications (adjusted OR 21.46, 95% CI: 8.86-51.97, p<0.001), a sheath external diameter/minimal femoral artery diameter ratio ≥1.05 (adjusted OR 4.52, 95% CI: 3.37-16.71, p=0.005), and female gender (adjusted OR 2.59, 95% CI: 1.26-5.31, p=0.010) were found to be independent predictors of major and minor vascular complications and percutaneous closure device failure (Table 4).
Discussion
The main finding of this retrospective study was that, in a large-volume and experienced TAVI centre, in patients undergoing transfemoral TAVI using either the 10 Fr Prostar XL or two 6 Fr ProGlide devices for common femoral artery haemostasis, we observed a significantly lower rate of vascular complications and percutaneous closure device failures in those cases where the Prostar was used. Of note, the use of the Prostar was not associated with reduced mortality, bleeding and stroke rates during hospitalisation.
In the transfemoral TAVI setting, percutaneous closure has been increasingly utilised over surgical cutdown7,10-12. The advantages of this less invasive technique are increased patient comfort immediately after the procedure and a diminished requirement for anaesthetic drugs during and after the procedure6,7. Suture-based closure devices have high success and very low vascular complication rates following percutaneous coronary angioplasty with 6-8 Fr sheaths13,14. In TAVI, larger calibre sheaths are used requiring a more careful closure. For this purpose, preclosure with either the Prostar or two ProGlide devices is widely used with good results7,8,10,12,15. However, whether one approach is superior to the other one has never been investigated so far. In this study we reported significantly lower rates of VARC-2 defined vascular complications and percutaneous closure device failure in patients where the large diameter femoral arterial sheath was removed by using the Prostar device.
Overall, arterial vascular injuries were more frequently represented by residual bleeding (persisting despite protamine administration and at least 10 minutes of manual compression) (42.3%) and flow-limiting dissection (29.6%), followed by common femoral artery occlusion (11.3%), residual critical flow-limiting stenosis (8.5%), and a combination of bleeding and dissection (7.0%). Considering a minimal effect of the learning curve as shown in Figure 3 (indeed, operators’ previous experience with both closure devices was remarkable, with hundreds of implants of each device performed before the study period), the reason behind this difference in terms of vascular complications and closure device failures may be the particular closure mechanism of these devices: the ProGlide device is advanced over a 0.035’’ guidewire and the first suture is deployed slightly angulated at 10 o’clock, while the second ProGlide device is inserted and deployed at 2 o’clock. The Prostar device requires a few minor expedients (subcutaneous tissue separation around the femoral artery, good pulsatile backflow from the cannula, etc.), but, when it is implanted correctly, it probably guarantees less traumatic deployment of the needles and more effective haemostasis once the sheath is removed. Hypothetically, we might speculate that the “foot” of the ProGlide manoeuvred into the vessel is a potential source of intimal dissection, and an incorrect angulation of the ProGlide before needle deployment could justify the higher rate of residual bleeding due to suboptimal suture of the vessel. Importantly, in our series, the vast majority of VCD failures or vascular complications were successfully managed without surgical intervention: stent implantation (56.0%), balloon angioplasty (32.4%) (Figure 5), or prolonged manual compression (8.0%) (Figure 6), with no differences between groups.
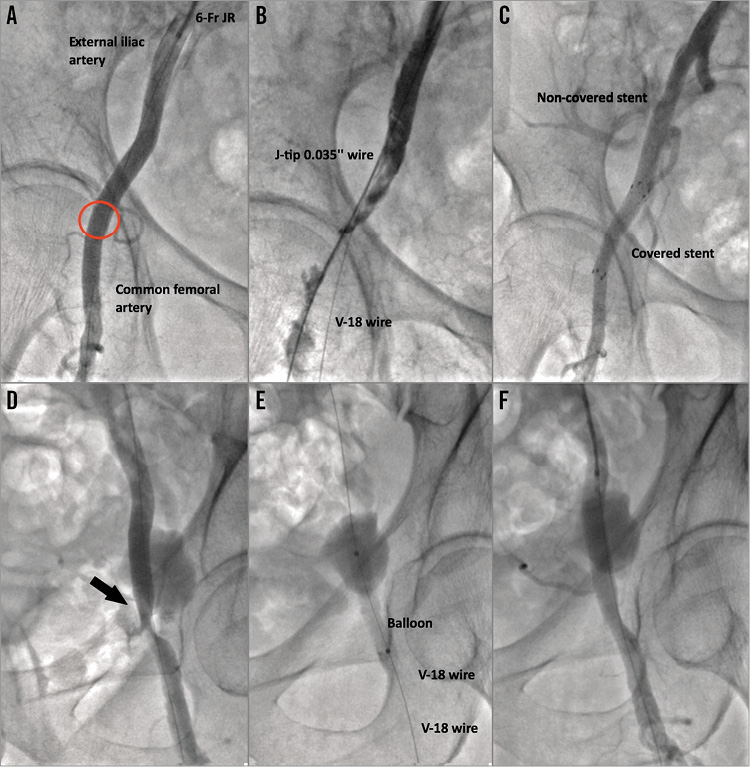
Figure 5. Case examples. A), B) & C) Treatment of common femoral artery injury with a Fluency Plus 6×40 mm covered self-expanding stent graft (Bard Canada Inc., Oakville, Canada). The red circle in panel A indicates the puncture site. D), E) & F) Treatment of common femoral artery injury (black arrow in panel D) with balloon angioplasty alone (Admiral Xtreme balloon; Medtronic Inc., Minneapolis, MN, USA).
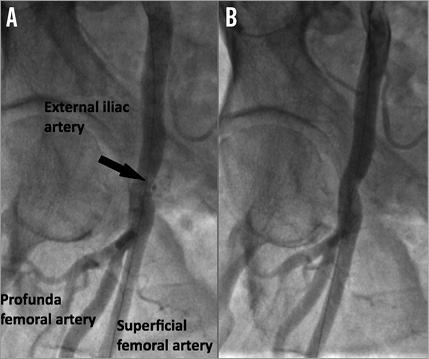
Figure 6. Case example. Successful treatment of common femoral artery injury (black arrow) with 20 minutes of manual compression. A) Selective femoral angiography showing residual bleeding. B) Selective femoral angiography showing the absence of residual bleeding after manual compression.
Surgical vascular intervention was required in 7% of cases, thus suggesting that the acute clinical impact of these complications was relatively modest. This concept is further underlined by the analysis of bleeding. In fact, although markedly higher rates of any vascular complications and percutaneous closure device failures in the ProGlide group were reported, we did not observe a statistically significant increase in bleeding rates and a consequent impact on other acute major clinical outcomes (i.e., mortality) in this study group. However, we cannot deny that a threefold increase in the common femoral artery stenting rate in the ProGlide group raises concerns, and it may potentially influence the operator’s device choice. The course of the common femoral artery through crossing flexion points (in this case, the hip region) potentially exposes the artery to relevant external forces, including compression, torsion, and elongation. Eventually, this may have a negative impact on stented vessel patency16-18. In fact, stent compression has been identified as one of the principal causes of frame fracture and restenosis, even in self-expanding nitinol stents17.
Along with the use of ProGlide vs. Prostar, in line with previous studies, a higher (more than 1.05) minimal femoral artery diameter to sheath outer diameter ratio7,8,10, moderate/severe iliofemoral calcification18, and female gender19,20 were also found to be independent predictors of vascular complications and percutaneous closure device failure in this analysis.
Finally, this study underlines the importance of checking the iliofemoral axes after sheath removal. Indeed, most of the vascular complications which subsequently required intervention were clinically silent, and they may have been undiagnosed if the iliofemoral angiography at the end of the procedure had not been performed. This was particularly relevant in the case of common femoral artery occlusion or residual flow-limiting dissection and stenosis. On the other hand, residual bleeding was easier to diagnose and subsequently to treat. However, what the outcomes of these lesions left untreated may have been remains unknown.
Limitations
This study has two main limitations. First, evaluating the impact of a specific VCD for transfemoral TAVI using a retrospective study can lead to incorrect conclusions because of the influence of unassessed confounding variables. In this study, each vascular closure approach was not assigned randomly, thus generating an unavoidable risk of bias regarding approach selection and the possible prognosis. However, VCD choice was not made according to specific criteria, but by alternating their use in order to maintain good expertise with both of them. This approach has generated two study groups with similar characteristics in terms of both clinical variables and anatomical features of the iliofemoral axis. The second limitation is the relatively small sample size, even though the present study represents the first attempt to evaluate acute comparative effectiveness of two ProGlide devices or one Prostar for vascular access closure during transfemoral TAVI.
Conclusions
In a large-volume and experienced TAVI centre, patients undergoing transfemoral TAVI had significantly lower rates of vascular complications and percutaneous closure device failures when the 10 Fr Prostar XL was used compared with two 6 Fr ProGlide devices for common femoral artery haemostasis. The use of the Prostar was not associated with reduced mortality, bleeding and stroke rates during hospitalisation.
| Impact on daily practice The development of the transfemoral TAVI technique in clinical practice has generated the need for VCD capable of accomplishing effective haemostasis after large diameter arteriotomies. This retrospective study tends to suggest that use of the Prostar device guarantees a more efficient haemostasis than use of two ProGlide devices in this setting. These results may potentially influence the operator’s device choice for common femoral haemostasis during TAVI. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

