Abstract
Background: Large-bore arteriotomy for transcatheter aortic valve implantation (TAVI) requires percutaneous vascular closure devices, but real-world data comparing different closure strategies are limited.
Aims: We sought to compare a dual ProGlide strategy vs a combination of one ProGlide and one FemoSeal for vascular closure after TAVI.
Methods: We retrospectively analysed 874 propensity score-matched patients undergoing TAVI at the Munich University Hospital from August 2018 to October 2020. From August 2018 to August 2019, a dual ProGlide strategy was used for vascular closure. From October 2019 to October 2020, a combination of one ProGlide and one FemoSeal was used. The primary endpoint was defined as access-related major vascular complications or bleeding ≥Type 2 according to Valve Academic Research Consortium 3 criteria.
Results: Patients in the dual ProGlide group (n=437) had a higher incidence of the primary endpoint than patients treated with one ProGlide and one FemoSeal (n=437; 11.4% vs 3.0%; p<0.001). Furthermore, they had a higher rate of closure device failure (2.7% vs 0.9%; p=0.044) and more often required unplanned surgery or endovascular treatment (3.9% vs 0.9%; p=0.004). The incidence of death did not differ significantly between groups (3.4% vs 1.6%; p=0.08).
Conclusions: A combined ProGlide and FemoSeal strategy might have the potential to reduce access-related vascular complications following TAVI.
Introduction
Transcatheter aortic valve implantation (TAVI) is the optimal therapy for patients with symptomatic severe aortic stenosis at high surgical risk1. Due to the results of the PARTNER 2 and 3 as well as the SURTAVI and Evolut Low Risk trials, the use of TAVI is increasingly extended to intermediate- and even low-risk patients2345.
Access-related vascular complications and bleeding remain the most frequent complications after transfemoral TAVI and are associated with worse short- and long-term outcomes467. Historically, suture-mediated percutaneous vascular closure devices (VCD) have been used for main access closure to avoid surgical cut-down. Among VCD, the Perclose ProGlide (Abbott Vascular) has shown superior results compared to the Prostar XL (Abbott Vascular) and has since become the most widely used suture-based VCD89. Additionally, a large-bore collagen plug-based VCD (MANTA; Teleflex) has been developed recently. Despite promising results in early feasibility trials and retrospective analyses, MANTA proved inferior to a dual ProGlide strategy in a recent randomised controlled study101112.
Initially proposed as a bailout strategy for excessive bleeding, a combination of suture-based VCD with additional collagen plug-based VCD has been reported to be safe and feasible1314. This approach theoretically reduces constriction of the common femoral artery and strain on the arterial wall while maintaining the advantages of both suture- and plug-based VCD. However, real-world data on vascular and bleeding outcomes of this approach are lacking.
Therefore, the objective of this study was to compare the use of a dual ProGlide technique (hereafter referred to as P+P group) and a combination of one ProGlide with the plug-based VCD FemoSeal (P+F group; Terumo) regarding vascular complications and bleeding in patients undergoing transfemoral TAVI.
Methods
In this retrospective single centre study, consecutive patients that underwent transfemoral TAVI from August 2018 to October 2020 at the Munich University Hospital were included.
From August 2018 to August 2019, vascular closure was performed using a suture-based strategy with 2 diagonally placed ProGlide systems. From October 2019 to October 2020, a combination of a single ProGlide system with a subsequently introduced FemoSeal system was used. Patients treated in September 2019 were excluded from this analysis to minimise the learning curve impact. In total, 1,018 patients underwent transfemoral TAVI during the selected time period. Twenty-nine patients were excluded due to primary use of a different closure device, conversion to open-surgery or death before access-site closure (Figure 1).
Figure 1. Study flowchart showing time period of inclusion. TAVI: transcatheter aortic valve implantation
All patients initially underwent contrast-enhanced computed tomography (CT) and transthoracic echocardiography in accordance with current European guidelines1516. TAVI was scheduled after obtaining consensus in the Heart Team. Transthoracic echocardiography and a duplex ultrasound of the main access site were performed routinely before discharge.
Patient data were collected from the electronic database that is part of the local EVERY VALVE registry (project number: 19-840) at the University Hospital Munich. The institutional ethics committee approved data acquisition and statistical analysis, and the study adhered to the tenets of the Declaration of Helsinki.
TAVI procedure
TAVI was generally performed under local anaesthesia. After the initial puncture of the femoral artery, a routine angiogram was done to confirm puncture height. In the P+P group, 2 VCDs were deployed diagonally (at 10 and 2 o’clock). In the P+F group, 1 ProGlide was inserted at the beginning of the procedure followed by the plug-based FemoSeal system at the end of the procedure. Intraprocedural anticoagulation was achieved with unfractionated heparin (50 to 70 IU/kg body weight) obtaining a target activated clotting time >250 sec. Manual compression was maintained until complete haemostasis was achieved.
Endpoints
The primary endpoint was defined as a composite of access-related major vascular complications or in-hospital bleeding ≥Type 2 according to the 2021 Valve Academic Research Consortium (VARC-3) criteria17. Secondary endpoints included overall vascular complications, closure device failure and bleeding according to the VARC-3 criteria, the need for unplanned surgery or endovascular treatment as well as the need for red blood cell transfusion. Additionally, standard procedural endpoints such as death, the need for a new pacemaker, stroke, unplanned revascularisation and acute kidney injury were obtained from the local registry.
Statistical analysis
Statistical analysis was performed using SPSS (version 25; IBM). The Student’s t-test and Wilcoxon rank-sum test were used to compare continuous variables as appropriate. The chi-square test was used to compare categorial variables. The normality of data distribution was assessed graphically. All tests were 2-sided and a p-value <0.05 was considered statistically significant. Propensity score matching was performed using the R package MatchIt (version 4.3.3; Ho, Imai, King, and Stuart) with a 1:1 nearest neighbour algorithm, no replacement, a 0.1 calliper and the following variables: age, sex, body mass index, estimated glomerular filtration rate, haemoglobin, diabetes mellitus, atrial fibrillation, history of stroke, left ventricular ejection fraction, oral anticoagulation, peripheral arterial disease, and chronic obstructive pulmonary disease18.
A logistic regression analysis was used to identify predictors for the primary endpoint. Both groups were divided into tertiles to exclude learning curve effects. Variables with p<0.1 were included in the multivariable analysis.
Results
Baseline characteristics
Baseline characteristics of the 989 unmatched and 874 matched patients included are presented in Table 1. Patients were well balanced except for a lower rate of chronic dialysis in the dual ProGlide group (0.5% vs 2.1%; p=0.033). Standardised mean differences are shown in Supplementary Table 1. Computed tomography characteristics of the main access vessel are depicted in Table 2. Tortuosity was lower in the dual ProGlide group (42.1% vs 51.0% ≥moderate tortuosity; p=0.017). Antithrombotic therapy is shown in Supplementary Table 1.
Table 1. Baseline characteristics.
|
Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
|
Dual ProGlide (n=491) |
ProGlide+FemoSeal (n=498) |
p-value |
Dual ProGlide (n=437) |
ProGlide+FemoSeal (n=437) |
p-value |
|
|
Age |
81.0±6.7 |
79.9±7.5 |
0.017 |
80.7±7.7 |
80.8±6.9 |
0.81 |
|
Female sex |
230 (46.8%) |
210 (42.2%) |
0.14 |
197 (45.1%) |
197 (45.1%) |
1 |
|
BMI |
26.5±5.2 |
26.6±4.8 |
0.61 |
26.6±5.2 |
26.4±4.7 |
0.75 |
|
Society of Thoracic Surgeons Score |
3.8±2.6 |
3.6±2.7 |
0.12 |
3.8±2.7 |
3.7±2.8 |
0.61 |
|
NYHA Class III or IV |
392 (80.0%) |
383 (76.0%) |
0.26 |
350 (80.1%) |
339 (77.6%) |
0.36 |
|
Diabetes mellitus |
140 (28.5%) |
118 (23.7%) |
0.08 |
118 (27.0%) |
107 (24.5%) |
0.40 |
|
Coronary artery disease |
297 (60.5%) |
303 (60.8%) |
0.91 |
259 (59.3%) |
261 (59.7%) |
0.89 |
|
Previous myocardial infarction |
69 (14.1%) |
64 (12.9%) |
0.58 |
57 (13.4%) |
54 (12.4%) |
0.76 |
|
Previous PCI |
164 (33.4%) |
158 (31.7%) |
0.57 |
145 (33.2%) |
138 (31.6%) |
0.61 |
|
Previous CABG |
41 (8.4%) |
28 (5.6%) |
0.09 |
36 (8.2%) |
24 (5.5%) |
0.11 |
|
Previous stroke |
66 (13.4%) |
62 (12.4%) |
0.64 |
59 (13.5%) |
56 (12.8%) |
0.76 |
|
Peripheral arterial disease |
53 (10.8%) |
47 (9.4%) |
0.48 |
48 (11.0%) |
46 (10.5%) |
0.83 |
|
Atrial fibrillation |
211 (43.0%) |
189 (38.0%) |
0.12 |
188 (43.0%) |
174 (39.8%) |
0.34 |
|
COPD |
51 (10.4%) |
66 (13.3%) |
0.16 |
48 (11.0%) |
46 (10.5%) |
0.83 |
|
Baseline eGFR |
49.1±20.0 |
51.7±22.9 |
0.06 |
49.6±20.0 |
50.0±20.4 |
0.85 |
|
Chronic dialysis |
5 (1.0%) |
10 (2.0%) |
0.20 |
2 (0.5%) |
9 (2.1%) |
0.033 |
|
Baseline haemoglobin level (g/dl) |
12.4±1.8 |
12.6±1.9 |
0.10 |
12.4±1.7 |
12.5±1.9 |
0.44 |
|
LV ejection fraction (%) |
50.8±8.4 |
51.6±9.4 |
0.18 |
51.1±8.1 |
51.3±9.6 |
0.79 |
|
Mean gradient (mmHg) |
37.0±13.3 |
36.3±14.0 |
0.40 |
35.1±13.2 |
36.4±14.4 |
0.49 |
|
Aortic valve area (cm2) |
0.75±0.21 |
0.76±0.21 |
0.32 |
0.75±0.20 |
0.76±0.22 |
0.69 |
|
BMI: body mass index; CABG: coronary artery bypass graft; eGFR: estimated glomerular filtration rate; LV ejection fraction: left ventricular ejection fraction; NYHA: New York Heart Association; PCI: percutaneous coronary intervention |
||||||
Table 2. Computed tomography characteristics of main access site.
|
Variable |
Dual ProGlide (n=437) |
ProGlide |
p-value |
|
|---|---|---|---|---|
|
Minimal lumen diameter (mm) |
7.7±1.8 |
7.9±1.8 |
0.23 |
|
|
Calcification |
None |
22 (5.0%) |
23 (5.7%) |
0.45* |
|
Mild |
162 (37.1%) |
153 (35.0%) |
||
|
Moderate |
212 (48.5%) |
216 (49.7%) |
||
|
Severe |
26 (5.9%) |
37 (8.5%) |
||
|
Vessel tortuosity |
None |
42 (9.6%) |
31 (7.1%) |
0.017† |
|
Mild (30-60°) |
194 (44.4%) |
175 (40.0%) |
||
|
Moderate (60-90°) |
120 (27.5%) |
145 (33.2%) |
||
|
Severe (>90°) |
64 (14.6%) |
78 (17.8%) |
||
|
Values are depicted as no. (percentage of total no.) *p-value refers to comparison of at least moderate calcification; †p-value refers to comparison of at least moderate tortuosity |
||||
Procedural results
Table 3 shows the procedural details. A radial access was more often used as secondary access in the P+P group (76.0% vs 65.4%; p<0.001). The mean sheath size was slightly, but significantly, larger in the P+P group (14.6±1.0 vs 14.3±0.8; p<0.001). The amount of contrast agent applied (122.2±68.7 vs 107.8±61.6; p=0.001) and fluoroscopy time (13.5±7.8 vs 12.4±7.4; p=0.032) were higher in the P+P group.
Table 3. Procedural details.
|
Variable |
ProGlide (n=437) |
ProGlide |
p-value |
|
|---|---|---|---|---|
|
Main access |
Right femoral |
392 (89.7%) |
399 (91.3%) |
0.42 |
|
Left femoral |
45 (10.3%) |
38 (8.7%) |
||
|
Secondary access |
Radial |
332 (76.0%) |
286 (65.4%) |
0.001 |
|
Femoral |
105 (24.0%) |
149 (34.1%) |
||
|
Sheath size (French) |
14.6±1.0 |
14.3±0.8 |
<0.001 |
|
|
Valve type |
SAPIEN 3 |
308 (70.5%) |
316 (72.3%) |
0.55* |
|
Evolut R |
53 (12.1%) |
65 (14.9%) |
||
|
Acurate neo |
73 (16.7%) |
56 (12.8%) |
||
|
LOTUS Edge |
1 (0.2%) |
0 |
||
|
Portico |
2 (0.4%) |
0 |
||
|
Balloon predilation |
215 (49.2%) |
210 (48.1%) |
0.76 |
|
|
Balloon post-dilation |
24 (5.5%) |
28 (6.4%) |
0.55 |
|
|
Percutaneous coronary intervention |
68 (15.6%) |
61 (14.0%) |
0.50 |
|
|
Procedure duration (min) |
44.6±22.0 |
42.3±19.9 |
0.11 |
|
|
Contrast agent (ml) |
122.2±68.7 |
107.8±61.6 |
0.001 |
|
|
Fluoroscopy time (min) |
13.5±7.8 |
12.4±7.4 |
0.032 |
|
|
Continuous variables are depicted as mean±standard deviation. Categorical variables are depicted as no. (percentage of total no.). *p-value refers to comparison of balloon-expandable valves vs self-expanding valves |
||||
In-hospital outcomes
The primary composite endpoint of main access-related bleeding ≥Type 2 or main access-related major vascular complications was significantly higher in the P+P group (11.4% vs 3.0%; p<0.001). Further, total vascular complications as well as total bleedings were significantly more frequent in the P+P group (Table 4). Patients in the P+P group had a higher rate of closure device failure (2.7% vs 0.9%; p=0.044) and more often required unplanned surgical or endovascular treatment (3.9% vs 0.9%; p=0.004). There were no differences in the rate of pseudoaneurysms (3.2 vs 3.4%; p=0.85). Details of the vascular complication type are depicted in the Central illustration and Supplementary Table 2. There was a trend towards a reduced mortality in the P+F group that did not reach statistical significance (3.4% vs 1.6%; p=0.08). The incidence of acute kidney injury, unplanned myocardial revascularisation, new pacemaker implantation and stroke were comparable between both groups (Table 4).
Table 4. In-hospital outcomes.
|
Variable |
Dual ProGlide (n=437) |
ProGlide +FemoSeal (n=437) |
p-value |
|---|---|---|---|
|
Primary endpoint* |
50 (11.4%) |
13 (3.0%) |
<0.001 |
|
Vascular complication – main access-related |
67 (15.3%) |
29 (6.6%) |
<0.001 |
|
Major |
43 (9.8%) |
12 (2.7%) |
<0.001 |
|
Minor |
24 (5.5%) |
17 (3.9%) |
0.27 |
|
Vascular complication – overall |
72 (16.5%) |
37 (8.5%) |
<0.001 |
|
Major |
48 (10.1%) |
15 (3.4%) |
<0.001 |
|
Minor |
24 (5.5%) |
22 (5.0%) |
0.76 |
|
Closure device failure |
12 (2.7%) |
4 (0.9%) |
0.044 |
|
Unplanned surgical or endovascular treatment |
17 (3.9%) |
4 (0.9%) |
0.004 |
|
Bleeding – main access-related |
69 (15.8%) |
22 (5.0%) |
<0.001 |
|
Type 1 |
22 (5.0%) |
9 (2.1%) |
0.017 |
|
Type 2 |
35 (8.0%) |
10 (2.3%) |
<0.001 |
|
Type 3 |
11 (2.5%) |
3 (0.7%) |
0.031 |
|
Type 4 |
1 (0.2%) |
0 |
1 |
|
Bleeding – overall |
104 (23.8%) |
35 (8.0%) |
<0.001 |
|
Type 1 |
29 (6.6%) |
13 (3.0%) |
0.011 |
|
Type 2 |
48 (11.0%) |
16 (3.7%) |
<0.001 |
|
Type 3 |
26 (5.9%) |
6 (1.4%) |
<0.001 |
|
Type 4 |
1 (0.2%) |
0 |
1 |
|
Need for red blood cell transfusion |
65 (14.9%) |
43 (9.8%) |
0.024 |
|
Delta haemoglobin (g/dl) |
−2.0±1.4 |
−1.9±1.3 |
0.49 |
|
Stroke |
14 (3.2%) |
6 (1.4%) |
0.07 |
|
Acute kidney injury |
56 (12.8%) |
48 (11.0%) |
0.40 |
|
AKIN 1 |
41 (9.4%) |
41 (9.4%) |
1 |
|
AKIN 2 |
5 (1.1%) |
2 (0.5%) |
0.45 |
|
AKIN 3 |
6 (1.4%) |
2 (0.5%) |
0.29 |
|
New onset of dialysis |
4 (0.9%) |
3 (0.7%) |
1 |
|
Unplanned revascularisation |
4 (0.9%) |
3 (0.7%) |
0.69 |
|
New pacemaker |
67 (15.3%) |
63 (14.4%) |
0.70 |
|
Death |
15 (3.4%) |
7 (1.6%) |
0.08 |
|
Continuous variables are depicted as mean±standard deviation. Categorical variables are depicted as no. (percentage of total no.). Vascular complications, closure device failure and bleedings are defined according to VARC-3 criteria. *Primary endpoint: composite endpoint of main access-related bleeding ≥Type 2 or main access related major vascular complication. AKIN: acute kidney injury network |
|||
In the multivariable analysis, age and coronary artery disease were independently associated with higher incidences of the primary endpoint (odds ratio 1.04; p=0.049 and 2.28; p=0.001, respectively), while the use of P+F was independently associated with lower incidences of the primary endpoint (odds ratio 0.24; p<0.001) (Supplementary Table 3).
Discussion
This retrospective single-centre study sought to compare 2 vascular closure strategies in a large real-world patient population undergoing transfemoral TAVI. The incidence of the primary endpoint of main access-related major vascular complications or bleeding ≥Type 2 was significantly higher in the dual ProGlide group than in patients treated with a combination of 1 ProGlide and 1 FemoSeal (Central illustration).
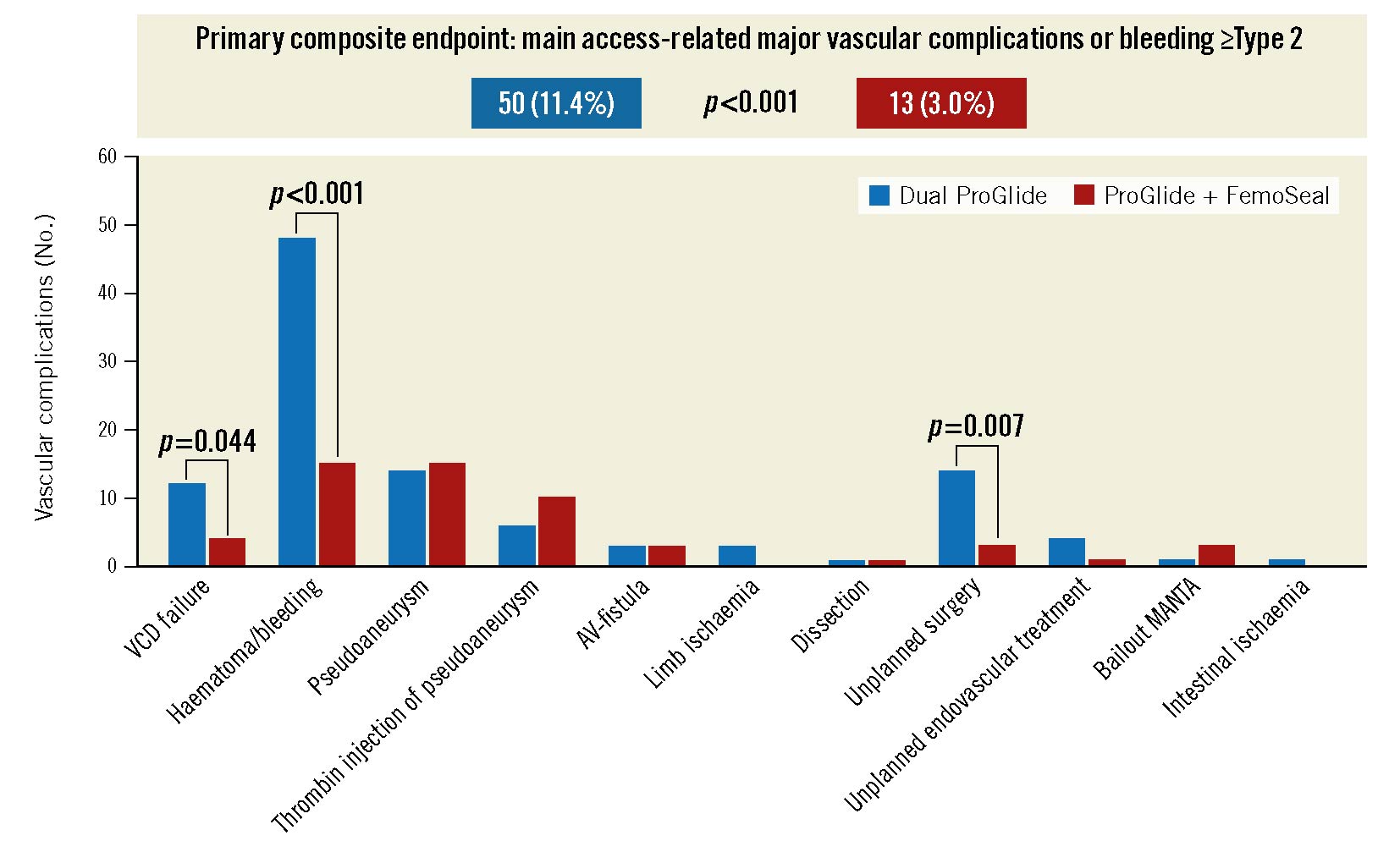
Central Illustration. Primary endpoint and vascular complications. >Minor and major vascular complications (absolute numbers) in both study groups stratified according to type of vascular complication. AV-fistula: arteriovenous fistula; Bailout MANTA: bailout strategy using the MANTA vascular closure device; Haematoma/bleeding: combined endpoint of VARC-3 bleeding and/or VARC-3 vascular complication due to haematoma; VCD: vascular closure device
Access-related vascular complications and bleeding remain the most frequent complications in patients undergoing TAVI and are associated with impaired outcomes467. For closure of the large-bore arteriotomy, traditional suture-based VCD have been most frequently used in clinical practice. However, the more recently developed large-bore plug-based VCD MANTA has been established as a widely used alternative strategy for vascular closure. Even though early feasibility trials and retrospective analyses showed promising results, the use of MANTA was associated with higher rates of vascular complication than a Dual ProGlide technique in 2 randomised controlled trials10111219. As an alternative, suture-based VCD can be combined with smaller sized plug-based VCD, e.g., AngioSeal (Terumo) or FemoSeal. Although initially proposed as a bailout strategy for closure device failure, this combination appeared to be safe and feasible in a smaller study by Ko et al14. In the recently published study by Costa et al, a combined approach reduced major vascular complications and bleeding20. However, the study was limited by a small sample size and heterogenic use of suture-based devices: the majority of patients were treated with 1 Prostar XL even though the ProGlide has proven to be superior89. One major concern when combining multiple VCD is constriction of the arterial lumen as described earlier with a consequent risk of peripheral ischaemia21. Hence, Ko et al combined a single ProGlide with one AngioSeal and even though no significant differences in overall vascular complications and bleeding were found, the authors reported a significantly lower rate of arterial stricture compared to a dual ProGlide approach. Nevertheless, larger studies comparing this hybrid technique to the standard dual ProGlide technique are lacking.
In our study, we compared the 2 strategies in a large real-world population at a tertiary European centre. In this setting, the primary endpoint of main access-related major vascular complications or bleeding ≥Type 2 was significantly higher in the dual ProGlide group than in patients treated with 1 ProGlide and 1 FemoSeal. Similar to a randomised controlled trial recently published by Abdel-Wahab et al and the study by Costa et al, this result was mainly driven by a high number of access bleeding and consequent haematomas, while the overall rate of other vascular complications such as arterial dissection or peripheral ischaemia was low in both groups1020. Nonetheless, the rate of unplanned surgical or endovascular treatments as well as the need for transfusion was significantly higher in the dual ProGlide group, implying clinical relevance of the observed complications. The rate of pseudoaneurysms was relatively high in this series of patients, which might be due to the systematic duplex ultrasound exam of the access site. However, routine ultrasound-guided puncture might reduce this rate. Major vascular complications and bleedings are known to be associated with increased mortality467. In our study, there was a trend towards higher mortality in the dual ProGlide group that did not reach statistical significance. However, retrospective analyses are prone to bias, and prospective studies are needed to confirm this observation.
As mentioned above, deployment of multiple ProGlide VCD significantly reduces the minimal vessel diameter. In our study, 3 patients in the dual ProGlide group underwent unplanned surgery for peripheral ischaemia, while this was not the case in the ProGlide+FemoSeal group. This finding is in line with the higher rate of arterial stricture in patients treated with multiple ProGlide VCD found by Ko et al and supports concerns of inducing haemodynamically relevant stenoses. In the published randomised controlled trials, 35-59% of the patients treated with a dual ProGlide technique needed additional VCD to achieve complete haemostasis1019. These additional VCD could reduce the residual arterial lumen and, hence, increase the risk of peripheral ischaemia even further. In our study, the incidence of closure device failure among patients treated with 1 ProGlide and 1 FemoSeal was low. Therefore, a combination of a single ProGlide with 1 small-sized plug-based VCD might, in fact, be advantageous, as it showed not only high efficacy but was associated with a reduced risk of subsequent peripheral ischaemia.
Compared to the randomised controlled study by Abdel-Wahab et al, we found a similar incidence of major vascular complications and bleeding ≥Type 2 in the ProGlide+FemoSeal group, but higher incidences in the dual ProGlide group. Rates of minor vascular complications or bleeding were lower in our study compared to the recent randomised controlled trials. We explain this with the retrospective nature of this study, as small haematomas without a relevant drop in haemoglobin might not be documented and, thus, remain undetected in retrospective analyses. However, these minor complications without clinical consequences are of questionable relevance.
Limitations
Even though propensity score matching resulted in equally balanced study groups, this is a retrospective analysis with its inherent limitations. All patients were treated at 1 large European TAVI centre. Further, there were some significant differences between the 2 groups. Main vessel tortuosity was higher in the P+F group. In contrast, the degree of calcification as well as the minimum lumen diameter of the main access vessel and the prevalence of peripheral arterial disease did not differ between groups, implying comparable vascular status. A radial access was more often used as secondary access in the dual ProGlide group. Finally, patients in the P+P group had a higher mean sheath size, which might lead to an increased bleeding risk.
Conclusions
The combination of suture-based with plug-based vascular closure devices might have the potential to reduce access-related major vascular complications and bleeding and, therefore, unplanned surgical or endovascular treatment in patients following TAVI.
Impact on daily practice
Vascular closure after transcatheter aortic valve implantation using a combined suture- and plug-based strategy (1 ProGlide and 1 FemoSeal) might result in reduced access-related major vascular complications and bleedings and, therefore, unplanned surgical or endovascular treatment, compared to an exclusively suture-based strategy (dual ProGlide).
Conflict of interest statement
M. Orban reports speaker honoraria from Abbott Medical, AstraZeneca, Abiomed, Bayer Vital, BIOTRONIK, Bristol-Myers Squibb, CytoSorbents, Daiichi Sankyo Deutschland, Edwards Lifesciences, and Sedana Medical; support for attending meetings from AstraZeneca; stocks from Abbott Laboratories, AbbVie, AstraZeneca, Bayer, Biontech, Bristol-Myers Squibb, Curevac, Draegerwerk, Fresenius Medical Care, Gilead Sciences, Inari Medical, Johnson&Johnson, Linde, Merck US, Moderna, NovoNordisk, Nuance Communications, Pfizer, Proctor&Gamble, Roche, SAP, Siemens Healthineers, and Zoom.D. Braun reports speaker honoraria from Abbott Vascular and Edwards Lifesciences.J. Hausleiter reports speaker honoraria and consulting fees from Abbott Vascular and Edwards Lifesciences. S. Deseive reports speaker honoraria from AstraZeneca. S. Peterß reports speaker honoraria from AstraZeneca. C. Scherer reports speaker honoraria from AstraZeneca. J. Mehilli reports institutional research grants from Boston Scientific and speaker honoraria from AstraZeneca, Pfizer, SIS Medical, Daiichi Sankyo. J. Steffen reports speaker honoraria from AstraZeneca and Travel support from theGerman Center for Cardiovascular Research (DZHK). The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

