
Transcatheter aortic valve implantation (TAVI) has become the standard treatment for patients with symptomatic severe aortic stenosis (AS) at elevated operative risk1,2, and new indications continue to emerge3. As the implementation of TAVI is expanding, controlling procedural complications is essential. Despite smaller profile devices and operator experience, recent trials have revealed that major bleeding and vascular complications are not uncommon, occurring in approximately 4-7% of cases4-6. These complications can frequently be attributed to inadequate vascular closure7. Moreover, this impacts on midterm and long-term clinical outcomes8.
The current standard for large-bore vascular closure is either surgical or with the use of suture-based closure devices, such as the Prostar® XL and Perclose ProGlide® (Abbott Vascular, Santa Clara, CA, USA). Currently, these conventional methods are being challenged by a new plug-based concept for large-bore vascular closure. The MANTA™ (Essential Medical Inc., Malvern, PA, USA) vascular closure device features a dedicated plug-based technology, consisting of a resorbable polymer and, unlike suture-based devices, it does not require pre-closure9. Possibly, the MANTA vascular closure device could reduce procedure-related complications, but it might also streamline the procedure since plug-based closure is supposed to be quicker.
In the current issue of EuroIntervention, Moriyama et al present a propensity-matched analysis of the ProGlide versus the MANTA vascular closure device10.
Herein, the authors touch upon a vibrant issue in the current TAVI era. From January 2016 to April 2017 they performed vascular closure with the ProGlide, after which they switched to default MANTA closure. In total, 330 patients were treated, which resulted in a cohort of 222 patients after 1:1 propensity matching. The use of the MANTA was associated with fewer major bleedings (10% vs. 20%, p=0.05) and fewer access site-related vascular injuries (8% vs. 17%, p=0.04). This was reflected in a more stable haemoglobin level after TAVI and a shorter hospital admission. Major vascular complications were remarkably low in relation to bleeding events and were not different between the two treatment arms.
The results of the study are appealing but far from conclusive and some important remarks should be made.
Propensity score matching has its inherent limitations/shortcomings. The authors chose to focus on baseline characteristics to perform the matching. However, one could argue that procedural characteristics should have been included in the matching process, since they also impact on the outcome. Moreover, the treatment arms were basically split by a time frame, namely before April 2017 and after. Consequently, potential predictors such as type of transcatheter heart valve or introducer sheath were unequally distributed. In particular, the imbalance in the large-bore sheaths used in this study complicates the interpretation and generalisability of the findings. The expandable sheath concept impacts on the arteriotomy size and subsequent closure device efficacy. Indeed, the 18 Fr MANTA should accommodate arteriotomies up to 22 Fr but the true arteriotomy after a 16 Fr eSheath™ (Edwards Lifesciences, Irvine, CA, USA) is inconsistent and may vary depending on the irregular shape of the eSheath upon removal. It is also remarkable that no 14 Fr MANTA systems were applied whereas the EnVeo R delivery system in-line sheath (Medtronic, Minneapolis, MN, USA) would be very compatible with the smaller MANTA. Hypothetically, use of the smaller MANTA may further reduce access-site complications given the smaller Fr size. Importantly, a final angiogram after access-site closure is missing. At this stage of comparing and adopting new large-bore closure technology a final contrast angiography in the catheterisation laboratory seems mandatory, not only from a research perspective but even more so from a clinical point of view. Closure device failure may result not only in overt bleedings but also in covert bleedings (i.e., in the retroperitoneal space), dissections and occlusions which should be actively excluded and reported.
In this study, the effect of the MANTA on major bleeding complications (risk reduction of 10%) can be considered high since, in most studies, major bleeding rates have not exceeded 10%. Consistently, the haemoglobin level dropped less in patients treated with the MANTA, which is confirmed by previous reports11.
Hospital stay was shortened by two days in the MANTA cohort. This is in line with another study which reported a one-day shorter hospital admission for patients treated with the MANTA compared to those treated with the Prostar XL11. MANTA closure might therefore not only limit procedure time but also streamline overall hospital admission.
At present, the published literature on the MANTA device for transfemoral access closure includes one prospective single-arm study and three retrospective non-randomised comparisons of MANTA. Figure 1 summarises the outcomes of major bleeding and vascular complications. In one prospective externally monitored single-arm study, 50 patients were all treated with the MANTA device which was followed by multiple follow-up visits12. The results were favourable, with a low rate of major vascular and bleeding complications (both one of 50 patients). This study paved the way for three retrospective comparative studies of MANTA versus either the ProGlide or Prostar XL. One study suggested an increased bleeding risk associated with the use of MANTA compared to ProGlide (8.0% vs. 2.7%)13, whereas one study showed a benefit of MANTA over Prostar XL (2% vs. 9%)11. In one study, major vascular and bleeding complications were similar for MANTA compared to ProGlide (9.3% vs. 12.2% and 15.9% vs. 16.5%, respectively)14. The results are conflicting, which can be partly attributed to the methodological set-up and sample size of these studies. The interpretability is hampered by the heterogeneity among the studies, which could be expected from these preliminary real-world data. Importantly, these studies were subject to potential bias since the populations in the respective treatment arms were inhomogeneous and were treated with different devices.
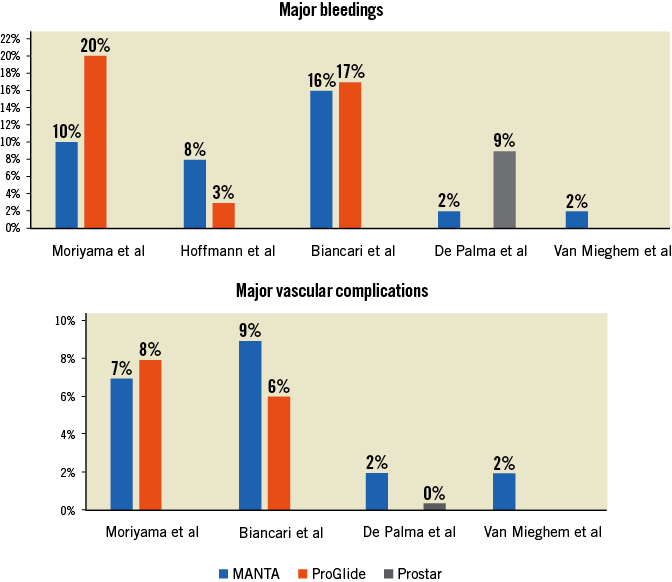
Figure 1. Major bleeding and vascular complications among previously published studies.
Are we ready to plug in?
What seems to resonate consistently with MANTA studies is the steep learning curve. Operators may become comfortable and adopt plug-based technology faster than suture-based platforms because plug-based designs are the most popular closure technology for small-bore arteriotomies (up to 8 Fr). Overall, data on short- and long-term clinical outcomes after vascular closure with MANTA are scarce. Prospectively collected data up to two months after the procedure showed vessel patency on ultrasound imaging and intact peripheral vascular perfusion reflected by unchanged ankle-arm indices12. The larger international “MANTA Registry for Vascular Large-bore Closure” (MARVEL) registry is currently enrolling up to 500 patients (ClinicalTrials.gov Identifier: NCT03330002) and will focus on major vascular complications and time to haemostasis after vascular closure with MANTA for both cardiac and peripheral interventions with large-bore access (10-18 Fr) in clinical practice.
In conclusion, the study by Moriyama et al confirms the arrival and growing adoption of plug-based MANTA technology in the large-bore arteriotomy scene. MANTA use seems safe in real life but whether it is superior to suture-based closure remains to be seen. It’s time to plug in for more data…
Conflict of interest statement
N.M. Van Mieghem reports receiving research grant support from Medtronic, Abbott, Boston Scientific and Essential Medical, and is on the Advisory Board for Essential Medical. The other author has no conflicts of interest to declare.

