
Abstract
Aims: Vascular and bleeding complications increase morbidity and mortality in transcatheter aortic valve replacement (TAVR). However, data regarding the efficacy and safety of the MANTA percutaneous vascular closure device (VCD) are scarce. The present study sought to compare VARC-2 complications between collagen plug-based closure using “MANTA” and suture-based closure using “ProGlide” to evaluate the efficacy of the novel MANTA VCD.
Methods and results: We performed a retrospective, propensity score-matched study to compare vascular and bleeding complications in 325 consecutive patients who underwent TAVR using MANTA and ProGlide. The 1:1 propensity score matching resulted in 111 matched pairs. For MANTA- versus ProGlide-treated patients, all-cause mortality (0% vs. 4%, p=0.02), vascular complications (14% vs. 21%, p=0.21), and bleeding complications (18% vs. 33%, p=0.01) were observed. Access-site vascular injury was significantly less frequent in patients who received MANTA versus ProGlide (8% vs. 17%, p=0.04). MANTA resulted in a significantly lower haemoglobin decrease (16.4 vs. 20.0 g/l, p=0.04) and shorter hospital stay after TAVR (3.3 vs. 5.8 days, p=0.02). It was also associated with fewer bleeding complications (OR 0.44, 95% CI: 0.23-0.83; p=0.01). Moreover, significant decreases of all endpoints were not seen across the procedure date tertiles in the MANTA group.
Conclusions: MANTA resulted in a significantly lower complication rate, especially for bleeding, than did ProGlide, despite the operators’ inexperience in the use of MANTA.
Abbreviations
AS: aortic stenosis
CT: computed tomography
OAC: oral anticoagulants
PS: propensity score
STS: Society of Thoracic Surgeons
TAVR: transcatheter aortic valve replacement
TF: transfemoral
THV: transcatheter heart valve
VARC-2: Valve Academic Research Consortium-2
VCD: vascular closure device
Introduction
Transcatheter aortic valve replacement (TAVR) is an established management strategy for severe aortic stenosis (AS)1-3. A transfemoral (TF) arterial approach using a suture-based preclosure technique is the prevalent manoeuvre. The preclosure technique using the ProGlide® (Abbott Vascular, Santa Clara, CA, USA) vascular closure device (VCD) overcomes the drawbacks of a surgical cutdown approach, such as general anaesthesia, longer hospital stay, and access-site complications. However, the complication rates of major vascular events as defined by the Valve Academic Research Consortium-2 (VARC-2) vary from 5 to 20% and the preclosure technique depends on the operator’s experience4-8. Moreover, based on a meta-analysis using newer-generation devices, the incidence of the critical bleeding complication rate is 3.9% (95% CI: 2.9-5.0)9. In contemporary randomised clinical trials, the frequency of life-threatening or disabling bleeding still ranges from 4.6 to >15%3,10. These complications remain associated with worse clinical outcomes after TAVR; further improvements are needed, even in the current era3.
A dedicated collagen plug-based VCD, the MANTA™ (Essential Medical Inc., Exton, PA, USA) for large-bore arteriotomy, is a novel method. Reports of some single-arm trials have revealed good results11,12. However, clinical data regarding MANTA are scarce. Therefore, the present study sought to compare VARC-2 complications between collagen plug-based closure using MANTA and suture-based closure using ProGlide to evaluate the efficacy of this novel VCD.
Methods
A total of 414 consecutive patients who underwent TAVR between January 2016 and March 2018 at Helsinki University Central Hospital were included. The inclusion criteria were TF-TAVR with ProGlide or with MANTA. One hundred and sixty-three consecutive patients received ProGlide between January 2016 and April 2017, while 162 consecutive patients received MANTA between April 2017 and March 2018. All TAVR were planned after the evaluation of computed tomography (CT). Two ProGlide devices, in a preclosure technique, were used before the femoral puncture13. MANTA has been described in detail previously11,14. Heparin reversal with protamine was mandatory before VCD use. Outcomes were reported according to the VARC-2 consensus15. The main outcomes were bleeding and vascular complications. Accordingly, early safety was assessed using composite endpoints. Composite endpoint 1 was defined as a composite of all-cause mortality, life-threatening or disabling bleeding, major bleeding, and major vascular complications. Composite endpoint 2 was defined as a composite of all-cause mortality, life-threatening or disabling bleeding, major and minor bleeding, major and minor vascular complications, and percutaneous closure device failure. Other outcomes were the duration of hospital stay and haemoglobin decrease (preoperative haemoglobin level - the lowest haemoglobin level after TAVR). Subsequently, these endpoints were chosen as markers of the learning curve of MANTA. Access-site or access-related injury was evaluated at the primary access site that was used for the TAVR large-bore sheath. In the learning curve analysis, patients were divided into tertiles based on the procedure date (1st tertile: n=37, April to July 2017; 2nd tertile: n=37, July to December 2017; 3rd tertile: n=37, December 2017 to March 2018). The Edwards eSheath (Edwards Lifesciences, Irvine, CA, USA) outer diameters for the 14 Fr eSheath for the 23 and the 26 mm transcatheter heart valve (THV), and the 16 Fr for the 29 mm were 7.65, 7.64, and 8.18 mm, respectively16. The 18 and 20 Fr LOTUS™ Introducer Sheath (LIS) (Boston Scientific, Marlborough, MA, USA) diameters were 7.4 and 7.9 mm, respectively. All clinical data were retrospectively determined from medical records. Written informed consent was obtained from all patients undergoing an elective procedure. The study protocol conformed to the Declaration of Helsinki.
STATISTICAL ANALYSIS
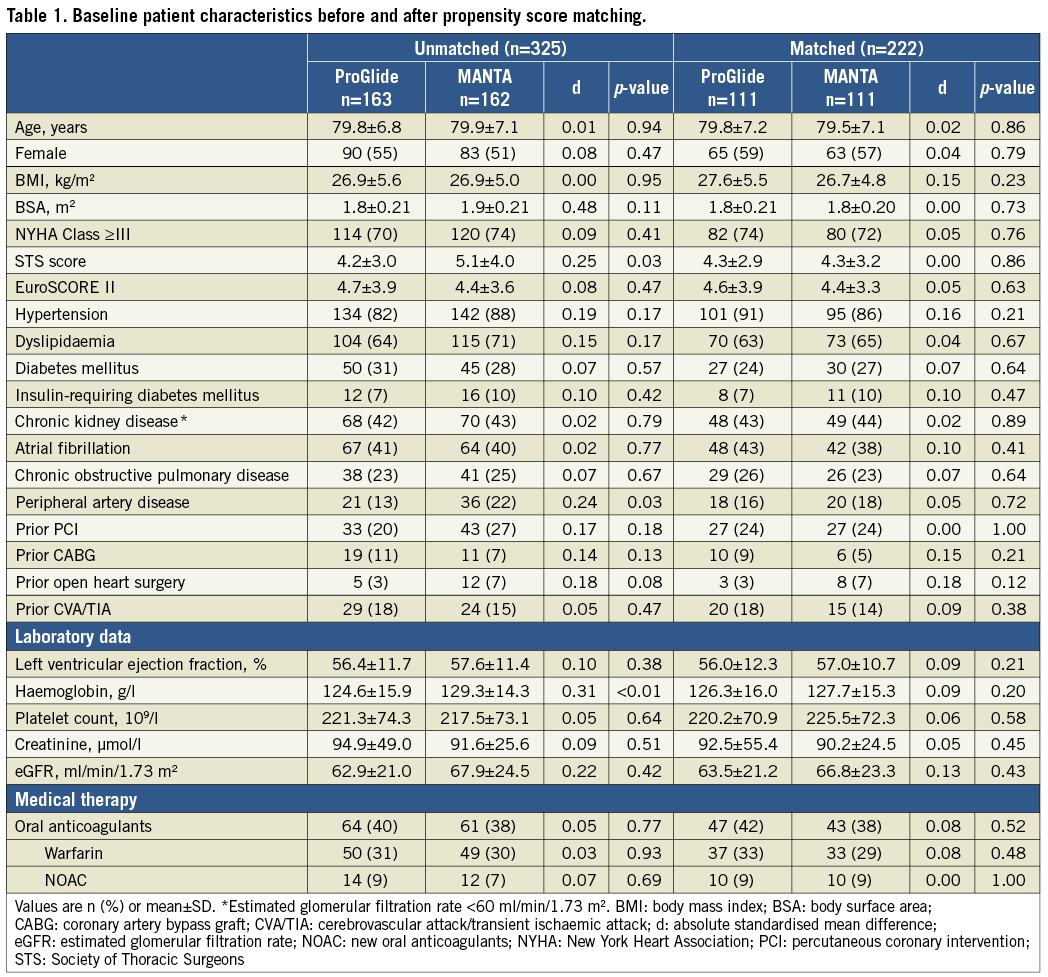
Categorical variables are presented as counts and/or percentages and were compared using the chi-square test. Continuous variables are presented as the mean±SD and were compared using the Student’s t-test or Wilcoxon rank-sum test on the basis of their distributions. A Cox proportional hazards regression analysis was used to obtain the odds ratio (OR) and 95% confidence interval (CI) for the development of each endpoint. Variables with p-values <0.1 on univariate analysis were selected for the multivariate model. A p-value <0.05 was considered significant. All statistical tests were two-tailed. Statistical analyses were performed using JMP10 (SAS Institute Inc., Cary, NC, USA). Because of the non-randomised nature of the study, we applied a propensity score (PS) matching method to adjust for confounding baseline variables. PS was modelled using a multivariate logistic regression model based on the following baseline characteristics: age, sex, body mass index (BMI), Society of Thoracic Surgeons (STS) score, EuroSCORE II, hypertension, dyslipidaemia, diabetes mellitus, chronic kidney disease, atrial fibrillation, chronic obstructive pulmonary disease, peripheral artery disease, left ventricular ejection fraction, use of oral anticoagulants (OAC), estimated glomerular filtration rate (eGFR), and haemoglobin level. A rigorous 1:1 nearest neighbour matching algorithm without replacement was attempted using a 0.2 calliper setting. Absolute standardised mean differences (d-values) were calculated and a d-value <0.2 was considered as the indicator of adequate bias reduction. PS matching was performed using SPSS, Version 22.0 (IBM Corp., Armonk, NY, USA).
Results
PS MATCHING AND PATIENT CHARACTERISTICS
A total of 330 patients underwent TF-TAVR. Five patients were excluded from the analysis, as shown in Figure 1. The remaining 325 patients successfully received either the ProGlide (n=163) or MANTA (n=162). The baseline characteristics of the ProGlide and MANTA patients are shown in Table 1. PS matching resulted in 111 matched pairs. After the matching, the absolute standardised mean difference (d-value) of all covariates was <0.2. This indicated an adequate balance of baseline characteristics (Supplementary Figure 1).
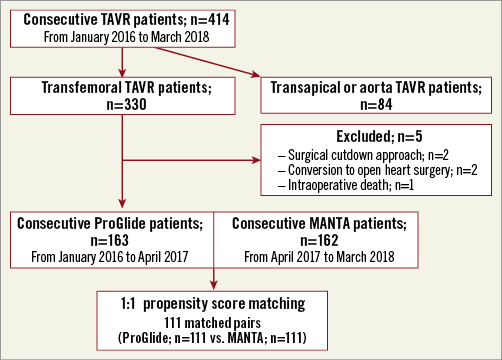
Figure 1. Study flow diagram. The incidence and independent predictors of VARC-2 outcomes were identified from 111 matched pairs of 330 consecutive transfemoral TAVR patients. TAVR: transcatheter aortic valve replacement; VARC-2: Valve Academic Research Consortium-2
PROCEDURAL CHARACTERISTICS
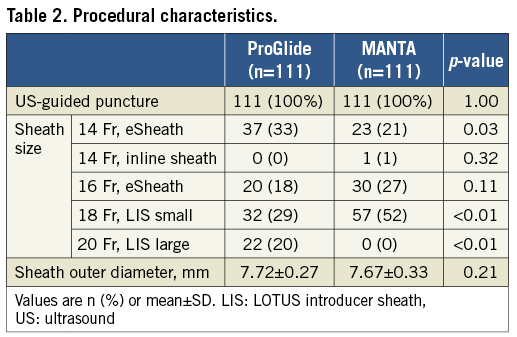
The procedural characteristics of the current study are presented in Table 2. LIS was applied for all cases of ACURATE neo™ (Boston Scientific), Evolut™ R (Medtronic, Minneapolis, MN, USA) and LOTUS implantation. The introducer sheath used was significantly different between the two groups (14 Fr eSheath: 33% vs. 21%, p=0.03; 18 Fr LIS, small: 29% vs. 52%, p<0.01, and 20 Fr LIS, large: 20% vs. 0%, p<0.01) but the sheath outer diameter was statistically similar between ProGlide and MANTA (7.72±0.27 vs. 7.67±0.33 mm, p=0.21). The THV differed significantly between the ProGlide and MANTA groups (Supplementary Table 1).
CLINICAL OUTCOMES AND COMPLICATIONS
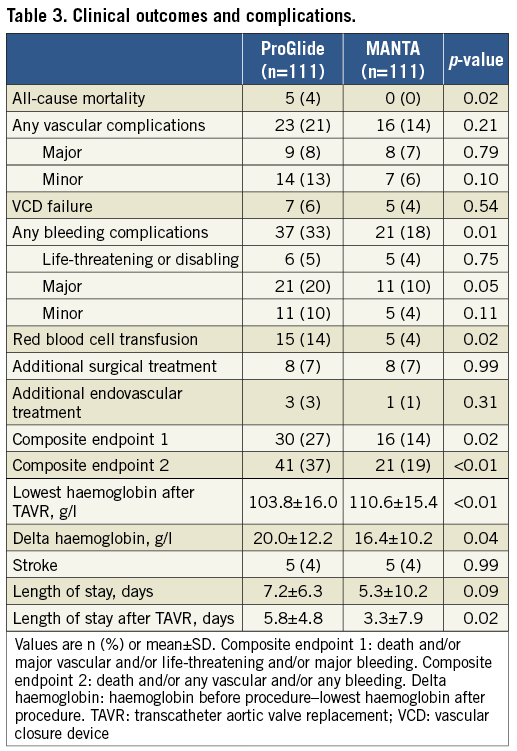
Clinical outcomes and complications are summarised in Table 3 and Table 4. VARC-2 vascular complications occurred less frequently in the MANTA group (14% vs. 21%, p=0.21) but the difference did not reach significance (Table 3). Access-site or access-related vascular injury was 17% in the ProGlide group and 8% in the MANTA group (p=0.04) (Table 4). VARC-2 bleedings were significantly less in the MANTA group (18% vs. 33%, p=0.01), especially in terms of major bleeding (10% vs. 20%, p=0.05). Accordingly, composite endpoints 1 and 2 were significantly different in both groups (composite endpoints 1 and 2 - ProGlide vs. MANTA: 27% vs. 14%, p=0.02, and 37% vs. 19%, p<0.01, respectively). Delta haemoglobin differed significantly between the two groups (ProGlide 20.0±12.2 vs. MANTA 16.4±10.2; p=0.04). Length of stay after TAVR was 5.8±4.8 and 3.3±7.9 days in the ProGlide and MANTA groups, respectively (p=0.02) (Table 3).
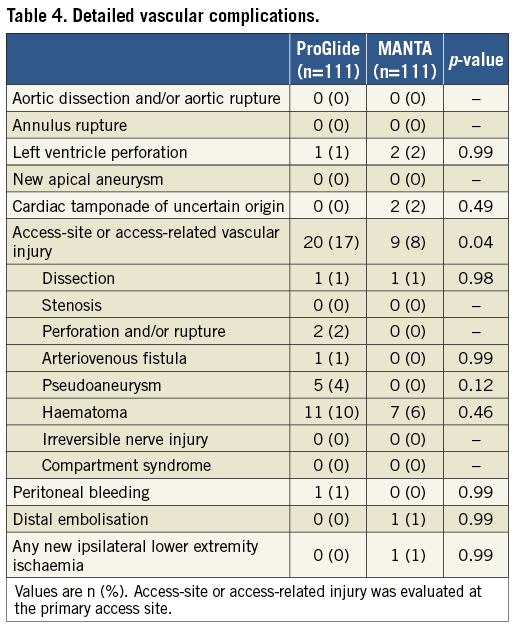
PREDICTORS OF VARC-2 COMPLICATIONS AND COMPOSITE ENDPOINTS
MANTA was identified as an independent predictor of freedom from VARC-2 bleedings (odds ratio [OR] 0.44, 95% CI: 0.23-0.83, p=0.01) and composite endpoints 1 (OR 0.46, 95% CI: 0.22-0.90, p=0.02) and 2 (OR 0.34, 95% CI: 0.18-0.65, p<0.01) (Table 5). Higher eGFR was also identified as an independent predictor of less frequent VARC-2 vascular complications (OR 0.63, 95% CI: 0.21-0.95, p=0.04), bleeding complications (OR 0.38, 95% CI: 0.09-0.67, p<0.01) and composite endpoint 2 (OR 0.42, 95% CI: 0.20-0.81, p=0.02) (Table 5). Although in the ProGlide cohort OAC was significantly associated with VARC-2 bleeding complications using multivariate analysis, it was not significant in the MANTA cohort (Supplementary Table 2). Differences in the incidences of each endpoint in the MANTA cohort were not observed in the OAC and non-OAC subgroups (Supplementary Figure 2). As shown in Supplementary Figure 3, the bleeding rate was higher in the ProGlide group with a larger sheath outer diameter. However, there were non-significant differences across the three tertiles of BMI and sheath outer diameter in the MANTA group.
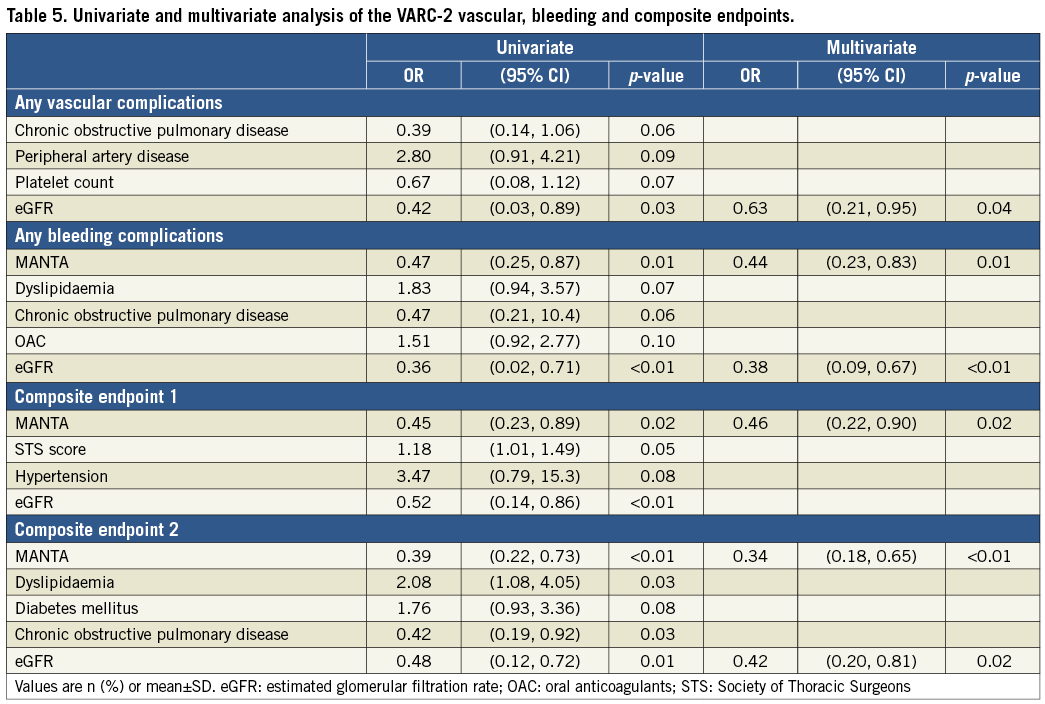
THE LEARNING CURVE FOR MANTA USAGE
Patient baseline characteristics across the three tertiles in the MANTA cohort were not significantly different except for dyslipidaemia (Supplementary Table 3). As shown in Supplementary Table 4, significant differences were not seen in any endpoints across the three tertiles. The scatter plot of haemoglobin decrease and of hospital stay also did not reveal a clear downward trend (Figure 2).
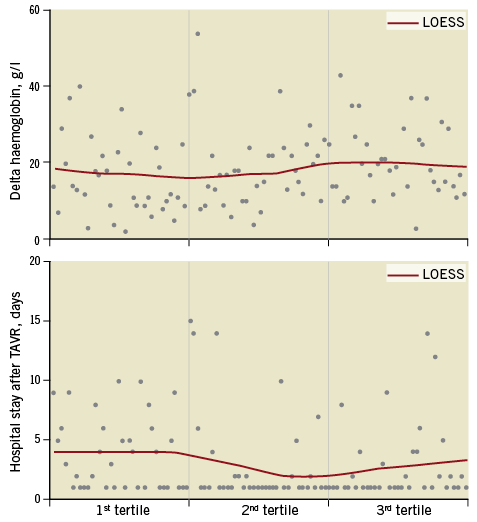
Figure 2. Scatter plot of haemoglobin decrease and hospital stay after transcatheter aortic valve replacement. Scatter plot does not show an obvious trend towards a decrease in these variables across tertiles. Delta haemoglobin: (haemoglobin level before TAVR)–(after TAVR). LOESS: locally estimated scatter plot smoothing
Discussion
The present study is a retrospective, propensity score-matched comparison of MANTA and ProGlide in patients who underwent TF-TAVR. The main findings are that: 1) the incidences of VARC-2 bleeding and of composite clinical complications were significantly lower in patients who received MANTA than in those who received ProGlide, 2) a lower rate of access-site and access-related vascular injuries was observed in the MANTA group, 3) MANTA usage was an independent predictor of fewer bleeding events, 4) a definite procedure-related learning curve was not observed for MANTA usage.
Although data regarding the MANTA are scarce, a previous report revealed few complications after MANTA deployment. A multicentre prospective study showed relatively low major vascular (2%) and major bleeding (2%) rates in 50 patients who received MANTA12. However, Biancari et al reported more frequent major vascular and bleeding complications (9.3% and 15.9%) in 107 patients17. The incidence rates of major vascular and bleeding complications after MANTA deployment in the current study were in the middle range compared to the values noted in the above reports. These results may indicate that further larger studies are needed to evaluate the efficacy of MANTA accurately.
Our study is the first to provide the outcomes of PS matching between patients who received MANTA and those who received ProGlide. The rate of any bleeding was significantly lower in the MANTA group. Importantly, the results are mainly derived from a significant reduction in major bleedings. Similarly, the decrease in haemoglobin level was also significantly less in the MANTA group. As previously reported, anticoagulant use was strongly related to bleeding events18. As expected, patients on OAC in the ProGlide group had a significantly higher incidence of bleeding events but, interestingly, there was no such tendency among patients on OAC and among the non-OAC patients in the MANTA group. It might be inferred that MANTA closes arteriotomy sites more appropriately than does ProGlide because of its collagen plug-based nature. However, previous reports regarding MANTA showed a tendency to increased bleeding events as the rate of OAC usage increased12,14,17. Therefore, the impact on bleeding events in patients who are to receive MANTA should be considered in a larger study.
In the current study, the occurrence of major vascular complications was statistically similar between the MANTA and ProGlide groups (7% vs. 8%, p=0.79). Biancari et al also reported that major vascular complications were noted in 9.3% of patients with MANTA and in 12.2% of patients with ProGlide (p=0.498)17. In all comparison studies between these VCDs, MANTA did not lower the occurrence of major vascular complications14,17. However, the incidences of access-site or access-related vascular injuries were significantly lower in the MANTA group (8% vs. 17%, p=0.045) in this study. Moreover, our data included four cases of a non-access-site-related vascular injury out of the seven cases of major vascular complications. In other words, the incidence of major vascular complications due to access or access-related vascular injury might be less frequent in the MANTA group. In addition to these findings, the length of hospital stay was significantly shorter in the MANTA group than in the ProGlide group (3.3±7.9 vs. 5.8±4.8 days, p=0.02). This may be mainly derived from less frequent bleeding events, including lower haemoglobin reduction, and less frequent access or access-related vascular injury. These results should be interpreted taking into consideration the operators’ inexperience with MANTA.
Various interventional procedures have a clear learning curve19,20. Suture-based vascular closure procedures, including ProGlide, have an obvious learning curve5. However, Van Mieghem et al reported reliable vascular haemostasis in the initial results of MANTA, despite the operators’ inexperience12. Major vascular complications were only 2%. Van Gils et al also reported similar findings in their initial experience with MANTA (major vascular complications: n=0)11. In the current study, downward trends were not seen in any endpoints across the three tertiles. This might indirectly indicate that MANTA usage does not require a learning curve, as did previous suture-based VCDs. However, our data were not powered to determine the exact learning curve because of the lack of procedural measures, such as time-to-haemostasis and procedure time.
Limitations
First, this was a retrospective single-centre study with typical limitations. Second, PS matching resulted in a sufficient balance of baseline characteristics; however, bias from unmeasured confounders could not be excluded because the patients were not randomised to the treatment groups. Moreover, we did not include procedural characteristics in the PS matching. This could have affected the results of the current article. Third, the sample size may be considered relatively small given that the efficacy of this device with regard to vascular complications could not be inferred. Fourth, the operators’ experience and procedural variables should have been included to evaluate the exact learning curve. Concerning the learning curve, our data indicated no more than a tendency. Fifth, we used an 18 Fr MANTA for all the patients, despite the sheath outer diameter. Therefore, these data do not evaluate the efficacy of the 14 Fr MANTA. Sixth, raw DICOM data between January 2016 and April 2017 were lost because of memory shortage of the server system. Therefore, no information on femoral vessel diameter, calcification, or on the ratio of the sheath diameter to the femoral arterial diameter was obtained21,22. Further analysis using CT variables of femoral arterial access may be useful to elucidate the predictors of vascular and bleeding events in patients who are to receive MANTA. Finally, we performed a separate logistic regression analysis on multiple endpoints. This might have introduced type 1 error to the results. However, even if the Bonferroni correction (p=0.0125) is applied, MANTA is still an independent predictor of fewer bleedings of any kind23.
Conclusions
Among a well-matched TAVR population, the rate of VARC-2 vascular complications was comparable between MANTA and ProGlide. However, the MANTA VCD strategy was associated with a lower rate of access-site or access-related vascular injury and VARC-2 bleeding complications, especially for major bleeding, despite the operators’ inexperience. Larger randomised studies are needed to elucidate our findings further.
| Impact on daily practice A dedicated percutaneous VCD for a large-bore TAVR sheath does not yet exist. MANTA is a reliable collagen-based VCD for large arteriotomies. Even given inexperience in its usage, MANTA can clearly reduce complications and even the length of hospital stay in patients who undergo TF-TAVR. Moreover, MANTA may have the potential to be safely applied in all patients regardless of obesity and OAC usage. |
Acknowledgements
The authors would like to thank Christina Salmen for her assistance in data collection.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary Figure 1. Propensity score matching.
Supplementary Figure 2. The incidence of clinical events in patients with or without oral anticoagulants.
Supplementary Figure 3. The incidence of clinical events according to body mass index and sheath outer diameter in patients with ProGlide and MANTA by tertiles.
Supplementary Table 1. Transcatheter heart valves.
Supplementary Table 2. Predictors of clinical outcomes according to the patients with ProGlide and MANTA.
Supplementary Table 3. Baseline and procedural characteristics in patients with MANTA by tertile.
Supplementary Table 4. Clinical complications and outcomes in patients with MANTA by tertile.
To read the full content of this article, please download the PDF.

