
Abstract
Aims: In the current study we assess the impact of two different access-site suture-mediated closure devices (SMCD), ProGlide and Prostar, on vascular and bleeding complications after transfemoral transcatheter aortic valve implantation (TAVI), as well as on long-term mortality.
Methods and results: From 2008 to 2013, 1,022 patients underwent transfemoral TAVI in two German centres using ProGlide (n=506) and Prostar (n=516) SMCD to close the access site. The primary outcome was the incidence of peri-TAVI major vascular complications according to Valve Academic Research Consortium-2 (VARC-2) definitions. Secondary outcomes were the incidence of bleeding complications and mortality. Compared to the Prostar SMCD group, patients in the ProGlide SMCD group less frequently experienced VARC-2 major vascular complications (7.5% vs. 15.9%, p<0.001), closure device failure (0.8% vs. 2.3%, p=0.04), any bleeding (BARC: 36.8% vs. 53.9%, p<0.001; VARC-2: 30.8% vs. 34.9%, p=0.59). Furthermore, one-year mortality was significantly lower in the ProGlide SMCD group, 14.8% vs. 19.5% in the Prostar SMCD group, log-rank p=0.04. However, VARC-2 major vascular complications but not ProGlide use were identified as an independent predictor of one-year mortality (adjusted odds ratio 1.54, 95% CI: 1.01-2.34 and 1.01, 95% CI: 0.65-1.55, respectively).
Conclusions: In this analysis, the use of ProGlide SMCD was associated with a reduced risk of vascular and bleeding complications following TAVI compared to Prostar SMCD usage. However, major vascular complications but not ProGlide use did independently predict long-term mortality. Clinical Trial Registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT02289339
Introduction
Together with reduction of paravalvular leakage after transcatheter aortic valve implantation (TAVI) seen with current prostheses, a decrease in delivery catheter entry profiles has resulted in a substantial increase in TAVI procedures performed via the transfemoral route1. The decrease of the sheath size has contributed substantially to a reduction of bleeding and vascular complications after TAVI2. However, the reported access-site vascular complications still range between 4% and 30% and are associated with longer hospitalisation, need for surgery, blood transfusion and increased mortality3-7. Therefore, the implementation of strategies to achieve an adequate haemostasis at the TAVI access site would be expected to increase the safety of these procedures further.
Two commonly available suture-mediated closure devices (SMCD) suitable for large vessel closure up to 21 Fr, the ProGlide® and the Prostar® systems (Abbott Vascular, Santa Clara, CA, USA), are most frequently used to achieve femoral access-site closure after TAVI. In contrast to open surgical closure, use of SMCDs allows access-site closure via a minimally invasive approach without direct visualisation of the vessel wall and permits a fully percutaneous TAVI procedure in the setting of sedation and local anaesthesia. Currently, studies addressing the in-hospital clinical safety and performance of these devices following TAVI have shown conflicting results8-13.
Thus, our study sought to assess and compare differences in performance between the ProGlide and the Prostar SMCD regarding vascular complications and long-term clinical outcomes in consecutive patients with symptomatic aortic valve stenosis (AS) undergoing transfemoral TAVI.
Methods
STUDY POPULATION AND DESIGN
Between January 2008 and December 2013, a total of 1,032 consecutive patients underwent transfemoral TAVI in two German centres, Munich University Clinic and Herzzentrum Bad Segeberg. Of these, 1,022 patients received SMCD to achieve minimally invasive access-site closure and were included in this study. Demographics, clinical and procedural data were collected prospectively as part of national quality control requirements and were documented in the dedicated database of our institution as part of the ongoing EVERY-TAVI registry. No informed consent for the institutional registry was required. Clinical follow-up was performed either by phone or in the outpatient clinic and was available for all patients at 30 days and for 98.7% of the patients at one year.
DEVICE DESCRIPTION AND PROCEDURAL DETAILS
The ProGlide closure device is a 6 Fr, two nitinol needle-guided suture-mediated closure system designed for closing the femoral artery access site in patients undergoing catheterisation procedures using sheaths ≤21 Fr. For sheath sizes >8 Fr, a double ProGlide technique is recommended. The Prostar closure device is a 10 Fr, four nitinol needle-guided device designed for artery access-site closure following procedures using 8.5-24 Fr sheaths. Details on the devices and closure techniques have been previously reported14. The decision about which SMCD to use was left to the operator. The Prostar SMCD was used exclusively until 2010 in the Herzzentrum Bad Segeberg and until 2012 in Munich University Clinic. Thereafter, all procedures were performed using the ProGlide SMCD. Since before the TAVI era the ProGlide SMCD was frequently used in both institutions to achieve access-site closure after transfemoral percutaneous coronary intervention (PCI), no overlapping period between the Prostar and the ProGlide SMCD was necessary.
Two types of TAVI prosthesis were implanted during this time – self-expanding CoreValve® prostheses (Medtronic, Minneapolis, MN, USA) and balloon-expandable SAPIEN XT valves (Edwards Lifesciences, Irvine, CA, USA). During implantation of self-expanding valves, a Check-Flo® Introducer (Cook Medical, Bloomington, IN, USA) – sheath external diameter (SED) of 7.2 mm – was used. For balloon-expandable valves, expandable eSheaths (Edwards Lifesciences) were used with expanded SED of 8.9 mm (for 23 mm and 26 mm SAPIEN XT valves) and 9.9 mm (for the 29 mm SAPIEN XT valve).
TAVI procedures were mainly performed under local anaesthesia. During TAVI, unfractionated heparin at a dose of 50-70 IU/kg of body weight or bivalirudin was given for anticoagulation following successful sheath insertion. Peri-TAVI antiplatelet treatment consisted of loading with 300 mg or 600 mg of clopidogrel, 75 mg clopidogrel daily for three months and 100 mg aspirin lifelong thereafter. Whenever oral anticoagulation was indicated, it was continued either as monotherapy or in combination with clopidogrel 75 mg daily for three months.
DEFINITIONS AND OUTCOMES OF INTEREST
The primary outcome was the incidence of major vascular complications at 30 days. Other outcomes of interest were the incidence of bleeding complications, all-cause mortality at 30 days and one year, as well as the incidence of closure device failure. Procedural events, vascular complications and bleeding complications were classified in accordance with the current Valve Academic Research Consortium-2 definitions (VARC-2)15. Additionally, bleeding complications were defined according to the Bleeding Academic Research Consortium (BARC)16. Since the VARC-2 definitions were published in 2012, event adjudication was performed retrospectively for patients enrolled before 2013. The event adjudication was performed by D. Jochheim and M. El-Mawardy and verified by J. Mehilli and M. Abdel-Wahab. Overall operator experience with SMCD use was defined as the number of days from the first SMCD usage.
STATISTICAL ANALYSIS
The population was divided into two groups according to the SMCD used – ProGlide and Prostar. Differences between the groups regarding the baseline characteristics and events were assessed for significance using the Student’s t-test or Wilcoxon rank-sum test (continuous data) or the chi-square or Fisher’s exact test where the expected cell value was <5 (categorical variables). Continuous data are presented as mean (standard deviation), while categorical data are presented as counts or proportions (%). Data distribution was tested for normality using the Kolmogorov-Smirnov test for goodness of fit.
To identify predictors of major vascular complications we performed a multivariable analysis including risk variables that showed an association with the dependent variable (p-value ≤0.10) at univariable analysis or had been reported in previous papers. Further, we estimated the survival curves with the Kaplan-Meier method and compared them with the log-rank test. Hazard ratios (HRs) of all events at 30 days and one year were calculated with Cox proportional hazards models. The assessment of the independent prognostic value of ProGlide use relative to the occurrence of one-year death was carried out by using a Cox regression model including all characteristics that differed between the two groups with a p≤0.1 in univariate analysis. Missing data in any of the variables considered in the multivariable analysis were ignored. In order to eliminate any differences created by increased user experience, we adjusted all multivariable models for the time since adoption of each closure device. A two-tailed p-value of <0.05 was considered to indicate statistical significance. Statistical software R-Statistics (version 3.1.0) was used for analysis.
Results
CLINICAL AND PROCEDURAL CHARACTERISTICS
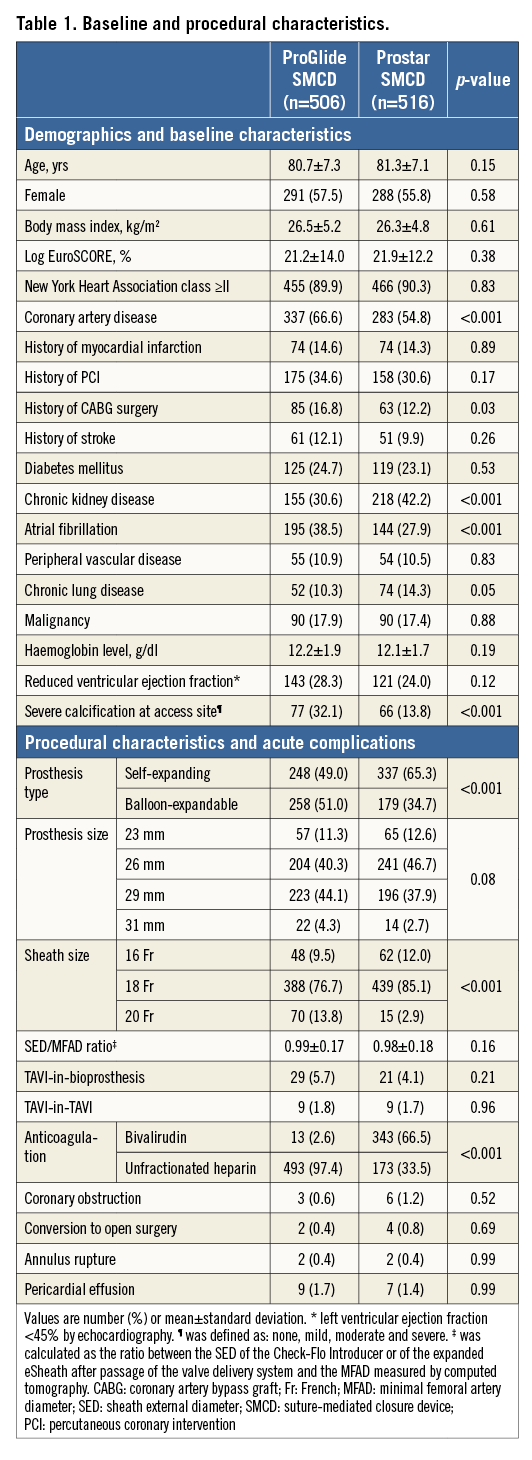
Out of the 1,022 patients undergoing transfemoral TAVI, the ProGlide SMCD was used in 506 (49.5%) while the Prostar SMCD was used in 516 patients. Baseline and procedural characteristics are shown in Table 1.
CLINICAL OUTCOMES
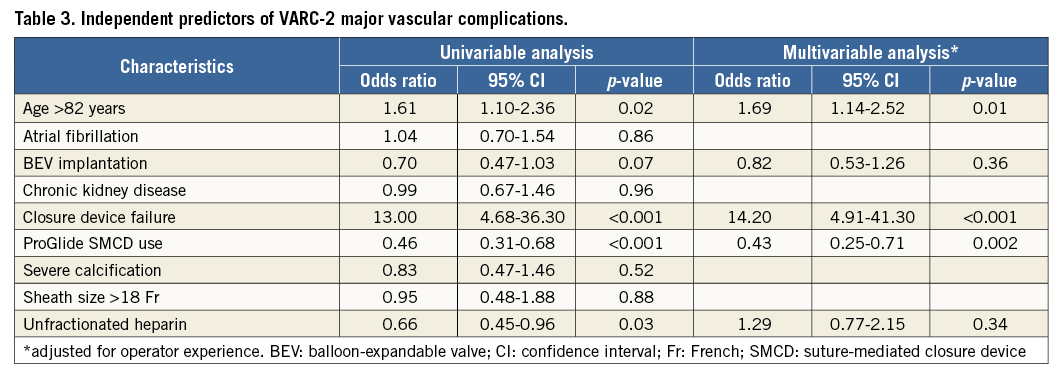
VARC-2 major vascular complications (the primary outcome) occurred more frequently in the Prostar group (p<0.001) (Table 2, Figure 1). Their incidence over time is shown in Figure 2. The distribution of different types of vascular complications was different between the groups (p>0.001) (Figure 3). In the multivariable analysis, older age, ProGlide SMCD use and SMCD failure remained as independent predictors of major vascular complications, even after adjustment for overall operator experience (Table 3).
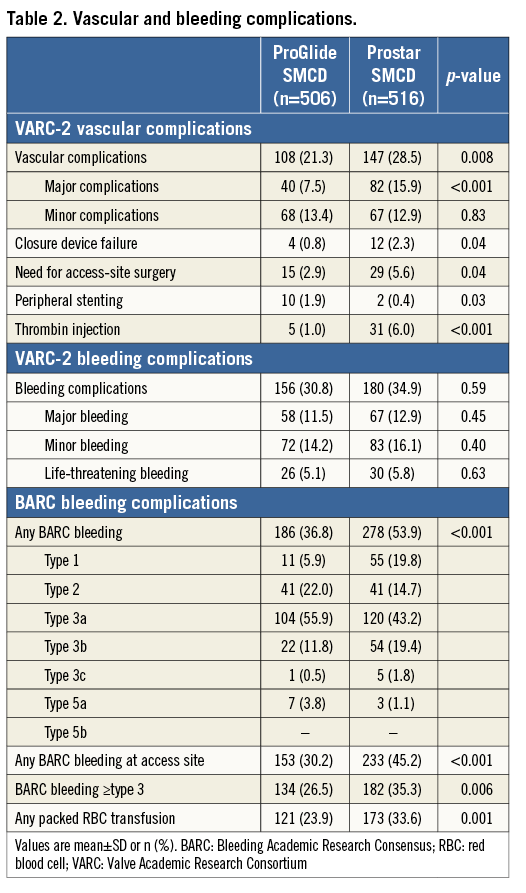
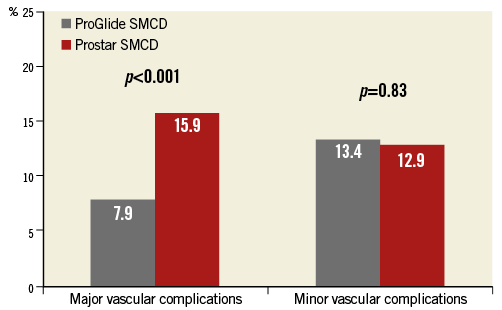
Figure 1. Major and minor vascular complications according to VARC-2 definitions.

Figure 2. Incidence of VARC-2 major vascular complications over time.

Figure 3. Distribution of various types of access-site vascular complications in both groups.
Compared to the Prostar group, the incidence of bleeding complications was significantly lower in the ProGlide group, when defined according to BARC classification (p<0.001) (Table 2), largely driven by less BARC type ≥3 bleeding observed in the ProGlide group (26.5% vs. 35.3% in the Prostar group, p=0.006).
There were no differences between the groups regarding all-cause mortality at 30 days (4.6% in the ProGlide group vs. 5.6% in the Prostar group, hazard ratio [HR] 0.62, 95% CI: 0.35-1.12). At one year, fewer patients had died in the ProGlide group (n=75) compared to the Prostar group (n=100) (cumulative incidence 14.8% vs. 19.5%, HR 0.73, 95% CI: 0.54-0.99) (Figure 4). In the multivariable analysis, chronic kidney disease (HR 1.43, 95% CI: 1.05-1.94), reduced LV ejection fraction (HR 1.71, 95% CI: 1.25-2.33), transfusion of any packed red blood cells (HR 1.91, 95% CI: 1.29-2.81) and VARC-2 major vascular complications (HR 1.54, 95% CI: 1.01-2.34) were identified as predictors of one-year mortality, while use of the ProGlide (HR 1.01, 95% CI: 0.65-1.55) played no part in it.
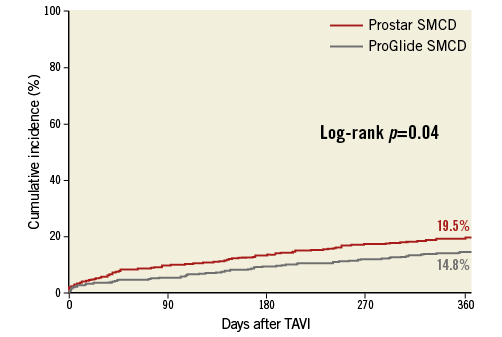
Figure 4. Mortality at one-year follow-up according to SMCD type.
Discussion
The current study is the largest one reporting the impact of two suture-mediated devices for access-site closure in a large cohort of consecutive patients with symptomatic severe aortic stenosis undergoing transfemoral TAVI on both peri-TAVI complications and long-term mortality. The main findings are: 1) use of the ProGlide SMCD is associated with a significantly lower incidence of vascular complications (particularly major ones) as well as bleeding complications compared to Prostar SMCD use; 2) use of the ProGlide SMCD is the only independent protective factor against major vascular complications; 3) however, although major vascular complications, among others, independently predict one-year mortality risk after transfemoral TAVI, use of the ProGlide SMCD does not.
Currently, more than 80% of TAVI procedures are performed via a transfemoral route, driven by the substantial reduction of delivery catheter entry profile of newer TAVI device systems as well as by the predominant use of suture-mediated closure devices to achieve adequate haemostasis at the femoral access site2,17,18. The fully percutaneous approach for transfemoral TAVI using SMCDs has been proven safe and effective and has become the predominantly used strategy to achieve access-site haemostasis5,11. Compared to a surgical cut-down for large femoral artery access-site closure, the use of a Prostar SMCD was associated with equal procedural success, shorter procedural time and time to ambulation in these patients19. Consistent with these findings, in the transfemoral cohort of the Placement of AoRTic TraNscathetER Valve trial, 85.7% of vascular complications occurred in patients undergoing TAVI via surgical cut-down17. The reported incidence of overall vascular complications with the Prostar SMCD device in TAVI patients ranges between 13% and 30%6,8,12,13,20. With the reduction of the sheath profile and increasing operator experience, the incidence of vascular complications with the Prostar SMCD has decreased1,7. In our Prostar SMCD cohort, the incidence of major vascular complications at 15.9% is comparable with rates reported by others (8% to 17%), considering the wide variety of definitions used for adjudication7,16,19,21. The majority of previous studies reported VARC-1-defined vascular complications and, as shown by Steinvil et al, the frequency of major vascular complications is doubled when applying the newest VARC-2 (current study) instead of VARC-1 definitions6.
With improving TAVI prosthesis platforms, the entry profile and the resulting sheath size continue to decrease, which in turn has made operators feel comfortable to use lower-profile SMCDs such as the ProGlide. However, to date there is only one randomised trial comparing the performance of two SMCDs, ProGlide and Prostar, versus open femoral exposure in 151 patients undergoing endovascular aortic aneurysm repair. The treatment success rate was highest (88% vs. 78% vs. 78%) and failure rates were lowest (6% vs. 12% vs. 10%) with the ProGlide SMCD compared to both the Prostar SMCD and open femoral exposure22. In our study, the incidence of VARC-2 major vascular complications was 7.5% with the ProGlide SMCD, which is identical to the rate observed in the recently published single-centre registry (8.0%)13. In the nine-centre CONTROL registry12, ProGlide SMCD use, as in our study, was associated with reduction of vascular complications compared to Prostar SMCD use; in the smaller single-centre Italian registry the opposite was reported. Diversity in the patient selection algorithm used to determine the TAVI access site in different centres, SMCD selection bias adapted to the access-site anatomy, particularly when both types of SMCD are used concurrently, and event reporting bias might explain the differences in the published complication rates and performance among the three different registries.
The overall SMCD failure rate was low but nonetheless three times higher with the Prostar SMCD (2.3%) compared to the ProGlide SMCD (0.8%) and, together with older age, independently predicted the increased risk of major peri-TAVI vascular complications. The 60% risk reduction observed with the ProGlide SMCD1,8 might be partially explained by lower device profile and the simultaneous two- instead of four-needle system.
According to the VARC-2 definition, the life-threatening and major bleeds (5.1% and 11.5%) observed in the current study are among the lowest published in the literature (4% to 20%)1,6,19,23. A central finding is the reduction of the clinically important BARC-defined bleeding complication (BARC bleeding ≥type 3) by nearly 30% in ProGlide SMCD patients. Administration of any packed red blood cells was also significantly reduced in the ProGlide group. This is an important finding, since blood transfusion has been identified as a strong predictor of mortality in patients undergoing TAVI or cardiac surgery23,24. In our analyses, VARC-2 major vascular complications together with reduced LV function, chronic kidney disease and blood transfusion independently predicted the risk of death at one year after TAVI. This might explain the 27% mortality reduction with the use of the ProGlide SMCD compared to the Prostar SMCD.
Limitations
In our study, mortality findings might have been influenced: i) by patient selection bias or the presence of unmeasured confounding factors despite multivariable adjustments; ii) by the sample size of 1,022 patients (adequate to compare performance of SMCDs but maybe not large enough to assess mortality differences); iii) by differences in valve technologies and learning curve experiences over time. To accommodate the operator’s learning curve with the Prostar SMCD, we did not consider TAVI procedures performed within the first year (2007); the ProGlide SMCD is a tool used in our centres for access-site closure after coronary interventions. However, the results remained unchanged after adjustment for overall operator experience in SMCD usage, and there was no time-dependent effect on vascular complications. Finally, considering the rapid uptake of a ProGlide SMCD instead of a Prostar SMCD pre-closure strategy in many centres, a randomised clinical trial to confirm the superiority of the ProGlide SMCD over the Prostar SMCD for reduction of peri-TAVI complications – even though desirable – will be difficult to perform. Therefore, the results of our study provide the strongest clinical evidence that use of the ProGlide SMCD to achieve haemostasis at the access site is safer and more effective than use of the Prostar SMCD.
Conclusions
In this analysis, use of the ProGlide SMCD was associated with a reduced risk of vascular and bleeding complications following transfemoral TAVI compared to Prostar SMCD usage. However, major vascular complications but not ProGlide use did independently predict long-term mortality.
| Impact on daily practice Use of suture-mediated closure devices (SMCD) to achieve access-site haemostasis during transcatheter aortic valve implantation (TAVI) permits a fully percutaneous procedure in the setting of sedation and local anaesthesia. Therefore, improvements of different closure techniques at the access site after transfemoral TAVI are of major importance for better outcomes. With ProGlide SMCD use, closure device failure and the risk of vascular and bleeding complications – both predictors of long-term mortality – are substantially reduced compared to Prostar SMCD usage. These findings suggest a safer fully percutaneous TAVI procedure using the ProGlide SMCD. |
Funding
The registry was supported by the Munich Heart Alliance partner site of DZHK.
Conflict of interest statement
J. Mehilli, J. Hausleiter and C. Kupatt report receiving lecture fees from Edwards Lifesciences. M. Abdel-Wahab reports receiving an institutional research grant from St. Jude Medical, and personal fees from Boston Scientific. G. Richardt reports receiving an institutional research grant from St. Jude Medical, and lecture fees from Boston Scientific and Edwards Lifesciences. The other authors have no conflicts of interest to declare.

