
Abstract
Aims: The success of transfemoral transcatheter aortic valve implantation (TAVI) and thoraco-abdominal aneurysm repair (TAAR) depends on haemostatic control of the access site, which is usually obtained with suture-based closure devices (Prostar or two ProGlide). A single ProGlide/Glubran technique, involving a suture placement on the vessel wall followed by tissue glue injection around the vessel wall, has not been previously investigated in this clinical setting. Our aim was to study the feasibility and safety of a single ProGlide/Glubran technique for vascular access-site closure after transfemoral TAVI and TAAR.
Methods and results: This technique was used in 250 patients from 2012 to 2017. The primary endpoint was the success of the technique, defined as access-site haemostasis without complications and not requiring any additional intervention within 30 days of the index procedure. Patients had a mean age of 82.4±1.93 years, with a logistic EuroSCORE of 20.2±2.32. A total of 218 TAVI and 32 TAAR procedures were performed with a mean sheath size of 18.09±1.55 Fr. The mean sheath to femoral artery ratio was 1.04±0.16, with mean femoral artery minimal lumen diameter 6.65±0.64 mm. The overall success rate of this technique was 98.4%. Four patients (1.6%) developed critical stenosis of the femoral artery requiring balloon dilatation. No major VARC-2 vascular complications were observed. Thirty-day mortality was 0.4% (non-cardiovascular).
Conclusions: The results of this study suggest that the single ProGlide/Glubran technique is a safe and effective method of closing the arterial access site after transfemoral TAVI and TAAR. The results of our study need to be confirmed in a randomised controlled trial before being adopted in routine clinical practice.
Abbreviations
AS: aortic stenosis
MLD: minimal lumen diameter
TAAR: thoraco-abdominal aneurysm repair
TAVI: transcatheter aortic valve implantation
VARC-2: Valve Academic Research Consortium-2
Introduction
Transcatheter aortic valve implantation (TAVI) and thoraco-abdominal aneurysm repair (TAAR) have emerged as therapeutic alternatives to an invasive surgical approach in patients with increased risk due to age and comorbidities1-7. Vascular and bleeding complications adversely affect early and late outcomes. Moreover, they also increase the length of hospital stay, leading to a higher cost burden8-10. A transfemoral (TF) route is preferred to deliver the transcatheter heart valve (THV) or the aortic endograft as it is associated with lower rates of mortality and vascular complications11.
Currently available transfemoral devices for these procedures require introducer sheaths/delivery systems ranging from 14-20 Fr for TAVI and 18-24 Fr for endovascular aortic graft prostheses. Suture-based devices are usually used to achieve haemostasis of these large sheath access sites and it is evident that increasing size of the delivery systems’ >19 Fr sheaths predisposes to higher rates of major vascular complications12. The Prostar XL® (Abbott Vascular, Santa Clara, CA, USA) suture-mediated system was reported to have a success rate similar to surgical cutdown in a meta-analysis for safety and efficacy in access sites requiring >10 Fr sheath insertion13. Further, it was observed to have a reduced procedure time compared to surgical cutdown. The Perclose ProGlide® Suture-Mediated Closure System (Abbott Vascular) was initially used for femoral artery access-site closure for sheaths 5-8 Fr during coronary intervention14 and, subsequently, a double ProGlide technique was widely adopted for access-site management during TAVI and TAAR with a high success rate15-17. However, the techniques described above have limitations in case of peripheral vascular disease, vessel calcifications, and obesity. The Glubran® 2 Seal device (GEM Srl, Viareggio, Italy) is a cyanoacrylate-based glue shown to be safe and effective in patients in these clinical settings when utilised in smaller sheath sizes18.
The use of a single ProGlide plus Glubran glue has not been previously investigated as an alternative strategy to achieve haemostasis of large sheath access sites. The rationale guiding this strategy is to reduce the size of the vascular access site to a diameter compatible with an 8 Fr sheath by using a pre-implanted ProGlide and achieving the final haemostasis by applying the glue on the arteriotomy site by means of the Glubran seal system.
The aim of our study was to assess the feasibility, efficacy and safety of ProGlide and Glubran for the haemostatic control of the arterial access site after TAVI and TAAR.
Methods
Study design and population
From January 2012 to June 2017, 254 (221 TAVI, 33 TAAR) consecutive patients who had undergone procedures with a totally percutaneous approach at the Policlinico di Monza, Italy, were studied. We included 250 patients in whom a single ProGlide/Glubran closure technique was used. This retrospective study design was reviewed and approved by the institutional review board. The indications for TAVI or TAAR were based on current guidelines19-22.
Exclusion criteria
Patients who were observed to have one of the following were excluded from the study: 1) CT findings for access site deemed unsuitable by the Heart Team, i.e., (i) significant stenosis or minimum luminal diameter (MLD) <4.5 mm, (ii) extensive calcification of the anterior wall of the femoral artery, (iii) aneurysms of the iliofemoral arteries and aorta, (iv) evidence of dissection and thrombus; 2) use of any vascular closure device in the past three months; 3) history of allergy to cyanoacrylate.
Preprocedural vascular assessment
Preprocedural evaluation for vascular access included contrast-enhanced computed tomography angiography (CTA) of the entire aorta and iliofemoral arteries to study the presence of significant vascular disease, thrombus, tortuosity of femoral and iliac arteries, and the MLD of the iliofemoral arteries. Calcification of the iliofemoral axis was graded by the extent of calcium on the circumference of the vessel at any point from aortoiliac bifurcation to the femoral artery at the level of the lower border of the femoral head (0-25% of circumference - 1, 26-75% of circumference - 2, >75% - 3). Tortuosity was defined by maximum angulation present along the iliofemoral axis and was graded from grade 1 to grade 3 as 0-60 degrees - 1, 61-90 degrees - 2, and >90 degrees - 3. Sheath to femoral artery ratio (SFAR) was calculated as the ratio of the outer diameter of the sheath used to deliver the device to the femoral artery MLD. The CTA images were assessed and quantified by both radiologists and cardiologists by means of a dedicated software (either OsiriX; Pixmeo SARL, Bernex, Switzerland, or 3mensio Medical Imaging BV, Bilthoven, the Netherlands) and subsequently discussed with the vascular surgeons during the Heart Team’s final discussion of each case. CT findings as described above were considered unsuitable for the femoral arterial approach.
Procedure
All procedures were performed under local anaesthesia and conscious sedation in the cardiac catheterisation laboratory. During each TF procedure, both femoral arteries were cannulated. To protect the femoral access selected for insertion of the delivery system, a crossover intraluminal 0.018’’ guidewire was placed in the superficial femoral artery (target) and the target access site was obtained under fluoroscopy to ensure puncture above the femoral bifurcation in the common femoral artery. Vessel puncture was performed using a standard Seldinger technique and a regular 0.035’’ guidewire was inserted. A small skin incision was made at the puncture site and a short 8 Fr sheath was temporarily placed and removed to dilate subcutaneous tissue. This facilitated advancement and correct positioning of ProGlide suture knots. After the pre-placement of the ProGlide, a short 8 Fr sheath was inserted using a standard 0.035’’ guidewire which was subsequently replaced by a 0.035’’ stiff guidewire through a 6 Fr diagnostic catheter.
At this point, a large-calibre sheath or the device’s delivery system (if a sheathless technique was used) was inserted under fluoroscopic guidance into the abdominal aorta. The procedure was then completed as per standard practice. At the end of the interventional procedure, the large-calibre sheath was removed along a standard guidewire. The closure was performed in two steps.
STEP 1
The operator carefully advanced the ProGlide knot to reduce the size of the vascular access site and an 8 Fr sheath was inserted (Figure 1). At this time, if we were able to achieve a complete clinical haemostasis (no visual bleed or increasing swelling around the access site with stable haemodynamics) with the 8 Fr sheath we proceeded to the next step; otherwise, we switched to a double ProGlide technique. The rationale for this step is that achieving good haemostasis with an 8 Fr sheath in situ implies a well applied successful ProGlide suture and vessel wall apposition around the 8 Fr sheath.
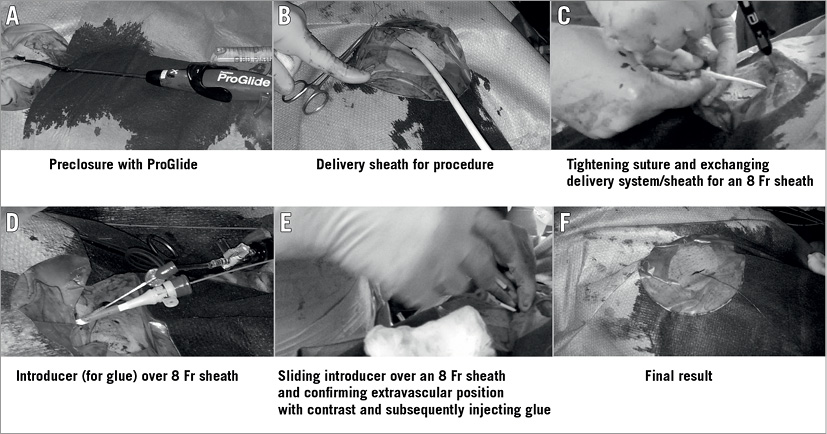
Figure 1. Stepwise illustration of the technique of ProGlide and Glubran glue injection. A) Standard preclosure with ProGlide. B) Procedure sheath/delivery system insertion. C) Larger sheath is exchanged for an 8 Fr sheath after tightening ProGlide suture (pre-placed). D) Introducer over the 8 Fr sheath. E) Glubran glue is injected under fluoroscopic guidance (after confirming extravascular position) to close the defect followed by manual compression. F) Final result.
STEP 2
This step involved completing the procedure by using cyanoacrylate glue (Glubran 2 seal). The Glubran 2 seal introducer is a radiopaque device approved for the closure of vascular access sites as wide as 8 Fr. It is made up of three parts: a proximal Luer-lock attachment, an intermediate steel pipe and a distal slide made of polypropylene. This distal part slides on the introducer sheaths, reaches the outside of the arterial wall and delivers the glue. No additional incision or space is required for the introducer to slide over the 8 Fr sheath as the subcutaneous space created for the delivery system is enough to allow its easy passage.
However, before the glue is injected, to avoid embolisation it is crucial to check by fluoroscopy the correct positioning of the seal device by injecting radiopaque contrast medium into the device and ensuring that the contrast diffuses external to the arterial wall rather than flowing into the artery. Once the seal device is deployed and its extraluminal position confirmed, the glue is injected, and the glue introducer and the sheath are removed together. Manual compression is applied, and anticoagulation reverted through protamine infusion (Figure 1A-Figure 1F, Figure 2).
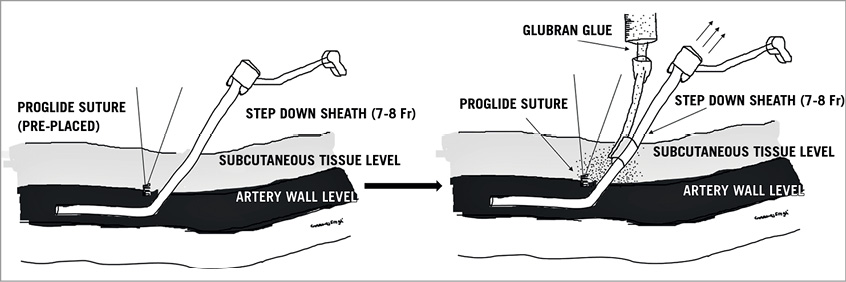
Figure 2. Schematic illustration of the technique of ProGlide and Glubran glue injection. A larger sheath is exchanged for an 8 Fr sheath after tightening ProGlide suture (pre-placed). Then Glubran glue is injected under fluoroscopic guidance to close the defect followed by manual compression.
A final crossover angiogram of the iliofemoral artery is crucial in all patients to ensure the achievement of complete haemostasis and to diagnose any complication. After the procedure, all patients were transferred to the intensive care unit for at least one night.
Endpoint definitions
The primary endpoint of the study was the success of the closure technique, defined as access-site haemostasis without complications and not requiring any further percutaneous or surgical intervention within 30 days of the index procedure. Complications were categorised according to the definitions of the Valve Academic Research Consortium-2 (VARC-2)23.
Statistical analysis
All patients’ data were handled with care to maintain patient confidentiality. Records are maintained in both computer and paper formats. Descriptive summaries are presented as frequencies and percentages for categorical data, and as means and standard deviations for continuous variables. All statistical analyses were performed using the SPSS statistical software package, Version 20.0 (IBM Corp., Armonk, NY, USA).
Results
Patient population
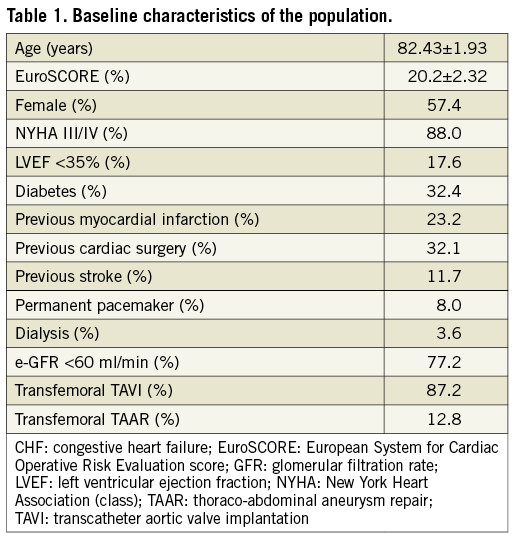
Two hundred and fifty-four consecutive patients were enrolled in the study. We switched to a double ProGlide closure technique in four patients and these were excluded from the analysis. The reason for excluding these patients was inadequate haemostasis following 8 Fr sheath insertion (with the application of suture) after exchanging with the delivery system, hence they were not considered suitable for the glue technique. None of the excluded patients had any factor related to vessel morphology (including extensive calcification or tortuosity) or vascular access technique that might have predisposed to a change of technique and thus exclusion from the study. The clinical characteristics of the patient population receiving the single ProGlide plus Glubran glue strategy are reported in Table 1. Two-hundred and fifty patients with a mean logistic EuroSCORE of 20.2±2.32 (147 women and 103 men, mean age 82.4±1.93 years) were treated; 32.4% of the patients had diabetes and 32.1% of the patients had a history of prior cardiac surgery. More than two thirds of the patients had an estimated glomerular filtration rate (GFR) of less than 60 ml/min.
Procedure details
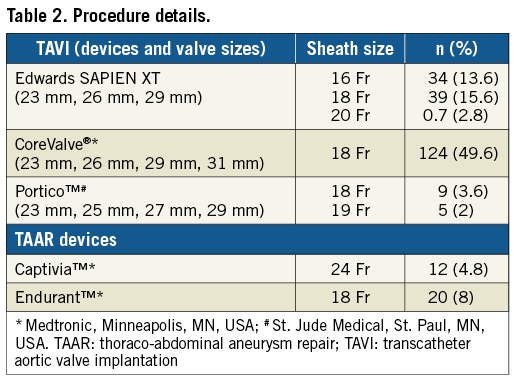
We included both TAVI (87.2%) and TAAR (12.8%) procedures with introducer sheaths of a mean size (±SD) of 18.09±1.55 mm. The devices and sheaths used are shown in Table 2. We used a wide range of sheath sizes in our study, ranging from 16 Fr to 24 Fr with outer diameters of 6.7 mm to 9.2 mm, respectively. Various access-site parameters are summarised in Table 3. Mean SFAR was 1.04±0.16. Severe calcification (>75% calcium on arterial wall circumference) was noted in 10% of the patients. Extremely tortuous vessels (>90 degrees angulation) were present in 5.6% of the patients.
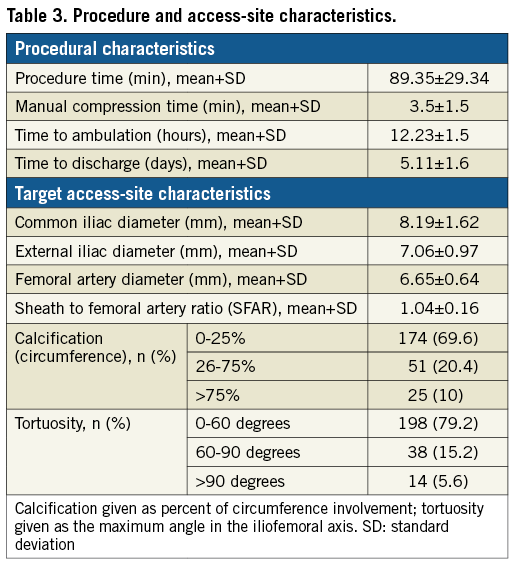
Study endpoints and complications
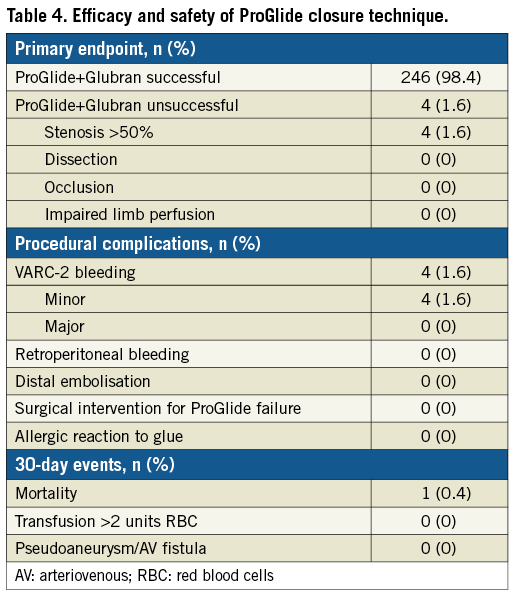
The overall success rate of the ProGlide+Glubran technique was 98.4%. In four patients (1.6%), it was necessary to perform a balloon dilation of the closure site, owing to angiographic evidence of critical stenosis after the percutaneous closure (Figure 3). Four patients (1.6%) had minor VARC-2 bleeding events which were managed conservatively. No VARC-2 major complications were experienced, and no patients were referred to surgery for vascular access complications (Table 4). The average time of manual compression was 3.5±1.5 minutes and mean procedure time was 89.35±29.34 minutes. Mean time to ambulation in our patients was 12.23±1.5 hours and the mean time to hospital discharge from the index procedure was 5.11±1.6 days. No in-hospital mortality was observed. At 30 days, one patient had died due to a non-cardiovascular cause (road traffic accident); consequently, the mortality rate was 0.4%. No additional VARC-2 bleeding complications occurred between the hospital discharge and 30 days post procedure.
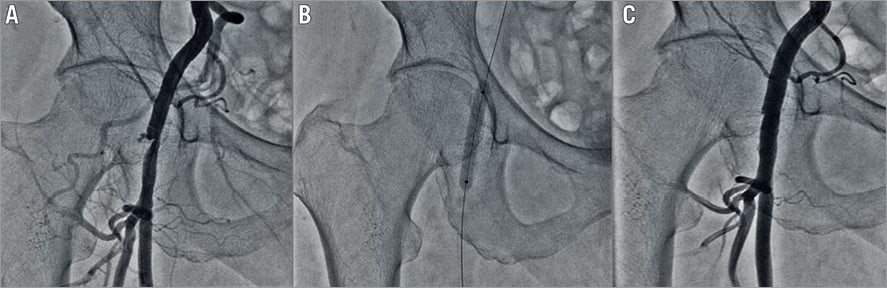
Figure 3. Illustrative case. A) A >50% stenosis post access-site closure with ProGlide and Glubran glue. B) Balloon dilatation of the stenosis with a peripheral angioplasty balloon. C) Final result.
Discussion
The result of this single-centre experience suggests that the ProGlide+Glubran technique is a feasible and safe strategy to achieve haemostasis after transfemoral procedures requiring large sheaths (≥14 Fr). Because of the technological advances and increasing operator experience, the benefit of performing transfemoral TAVI or TAAR is becoming increasingly evident in terms of procedural success and clinical outcomes. Procedural complication rates can be affected by the strategy chosen to achieve access-site haemostasis. Suture-based closure techniques have been used extensively for large arteriotomy closures following transcatheter percutaneous interventions. Although the double ProGlide is currently one of the most commonly adopted methods for management of large access sites, multiple ProGlide devices have been used in the past for haemostatic control24. A single ProGlide technique has also been studied to achieve successful haemostasis for large-calibre sheaths in both TAVI and TAAR patients15,25. It was found to have comparable outcomes to double ProGlide in terms of technical success as well as similar bleeding events in patients of the OCEAN-TAVI trial26. However, the observation that haemostasis may not be completely achieved with the use of a single ProGlide is a common clinical experience.
In our study, we combined the single ProGlide technique with the glue, an action aimed at achieving optimal haemostatic control of the access site. The success of achieving haemostasis may be attributed to two different levels of action, at the vessel wall level (suture) and subcutaneous tissue level (glue). The glue used in this technique was reported to be safe and effective in closing femoral puncture sites (5-8 Fr) in patients with peripheral artery disease with a high success rate (96.1%)18. The addition of glue to the pre-placed suture was successful in achieving haemostasis even in heavily calcific and tortuous vessels in our patient population.
The success rate of the ProGlide+Glubran technique is comparable with other major devices/methods reported in the literature for the management of femoral access sites after TAVI or TAAR (i.e., double ProGlide and Prostar XL). The benefit of a percutaneous approach for procedures involving larger access sites is evident from the reduced procedural time, time to ambulation and time to discharge when compared to surgical cutdown13. In the CONTROL study27, Prostar and double ProGlide were compared for vascular complications. Different bleeding patterns were attributed to double suture failure leading to higher major bleeding events in the Prostar group (16.7 vs. 3.2%, p<0.001; OR 6.33, 95% CI: 3.45-11.64) and single suture failure in the ProGlide group with higher minor bleeding events. Double ProGlide use was also seen to have a higher rate of arterial stenosis. Use of a single suture in our study led to lower rates of arterial stenosis (1.6%). A recent systematic review of patients who underwent endovascular aortic aneurysm repair (EVAR) or TAVI found greater bleeding event rates with Prostar as compared to double ProGlide, without a difference in mortality, acute kidney injury or total vascular complications28. We observed a few minor bleeding events which could be managed conservatively. Hayashida et al29 reported that an SFAR ratio of more than 1.05 predicted major VARC bleeding. Although we had a mean SFAR of 1.04±0.16, we did not experience any major bleeding events. In addition, we used this technique successfully covering a wide range of sheath sizes (16-24 Fr).
We attribute this success to the two steps followed in this procedure. Firstly, after exchanging the delivery system for an 8 Fr sheath, we ensured that there was adequate clinical haemostasis. Secondly, all patients had a control angiogram after completing the closure of the target access site from the contralateral side.
There are some additional benefits of this technique. Firstly, it is likely to be learned quickly, as both ProGlide and cyanoacrylate glue individually may be used in routinely performed coronary or peripheral interventions. Secondly, using multiple devices theoretically predisposes to arterial stenosis which is less likely to happen with this technique. Thirdly, this glue introducer is a low-profile device (not requiring further dilatation for sliding over the sheath), and introduction does not depend on the angle of insertion or meticulous placement of the second suture. Fourthly, it is likely to decrease the overall cost of the procedure along with the benefit of early ambulation and discharge.
Study limitations
Our study is a single-centre, non-randomised retrospective analysis without comparison to currently used methods. The number of events was too small to allow us to perform multivariate analysis and identify predictors of ProGlide/Glubran failure and/or vascular complications.
Conclusions
Our study suggests that transcutaneous closure of femoral access sites by means of a single ProGlide plus Glubran cyanoacrylate glue technique after TAVI or TAAR is safe and effective. The results of the study suggest the prospect of designing a randomised controlled trial of comparison between the ProGlide+Glubran technique and the currently used approach to achieve haemostasis after transfemoral endovascular procedures requiring large sheaths (≥14 Fr).
| Impact on daily practice This study makes the approach to percutaneous procedures involving large-calibre delivery systems simple, safe and effective. This may facilitate increasing the number of these procedures performed in a greater number of centres around the world, overcoming the reluctance which arises due to vascular complications. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

