Abstract
Aims: The objective of this study was to describe and evaluate the adjunctive technique of Angio-Seal (AS) use to augment the dual Perclose ProGlide (PP) in achieving haemostasis in patients undergoing transfemoral percutaneous transcatheter aortic valve replacement (TAVR).
Methods and results: All patients who underwent TAVR from May 2007 to January 2015 via a planned transfemoral percutaneous approach with a dual PP pre-close strategy were retrospectively analysed. This cohort was divided into two groups: dual PP versus dual PP with adjunctive AS (PP+AS) use based on the specific status of intraprocedural haemostasis. The baseline and procedural characteristics and in-hospital outcomes were prospectively collected and retrospectively compared. Overall, a total of 387 consecutive patients (55% male, mean age 83 years) with dual PP (n=179) vs. dual PP+AS (n=208) were evaluated. There were no statistically significant differences between the dual PP vs. dual PP+AS groups with regard to the in-hospital Valve Academic Research Consortium-2 (VARC-2) primary endpoints of major vascular complications (8.0% vs. 6.6%, p=0.592), minor vascular complications (18.4% vs. 13.7%, p=0.218), life-threatening or disabling bleeding (5.1% vs. 3.0%, p=0.291), major bleeding (1.7% vs. 1.5%, p=1.000), and minor bleeding (14.4% vs. 10.6%, p=0.271).
Conclusions: The adjunctive Angio-Seal technique to augment the dual PP pre-close strategy for patients undergoing percutaneous femoral closure following TAVR is feasible and safe and may be considered as a bail-out or an alternative strategy when the dual PP closure technique fails to obtain complete haemostasis.
Abbreviations
AS: Angio-Seal
CBOT: crossover balloon occlusion technique
PP: Perclose ProGlide
STS: Society of Thoracic Surgeons
TAVR: transcatheter aortic valve replacement
THV: transcatheter heart valve
VARC-2: Valve Academic Research Consortium-2
Introduction
Femoral arteriotomy closure following transcatheter aortic valve replacement (TAVR) remains challenging and, at times, may result in significant or even fatal complications1-4. Despite the gradual increase in the less invasive, percutaneous approach in clinical practice, complications associated with the femoral access site are reported in 5-20% of patients undergoing TAVR1,5-7. These complications have been associated with significantly increased patient morbidity, mortality and hospitalisation rates1-4. The incidence of access site-related vascular complications reinforces the need for the improvement of techniques that ensure effective and reproducible haemostasis with a safe and effective arteriotomy closure. The Perclose ProGlide (PP) suture-mediated closure device (Abbott Vascular, Santa Clara, CA, USA) has been widely utilised in a dual pre-close strategy8. Nevertheless, failure of this device requiring further intervention has been described in 4-9% of cases1,5,7,9. The objective of this study was to describe a modified, vascular closure technique, which utilises the Angio-Seal (AS) device (St. Jude Medical, St. Paul, MN, USA) as an adjunctive measure to augment the dual PP pre-close approach in achieving improved haemostasis in selected cases.
Methods
PATIENT POPULATION AND DATA COLLECTION
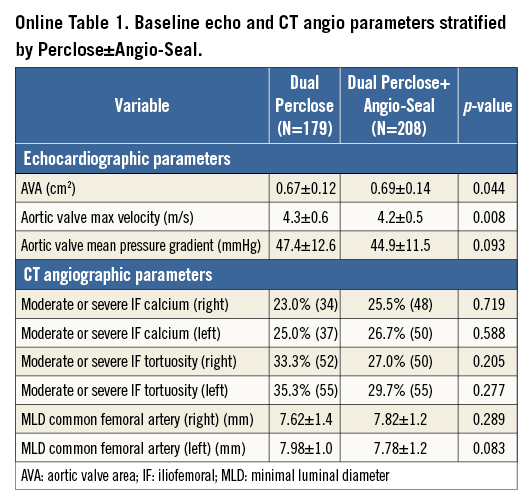
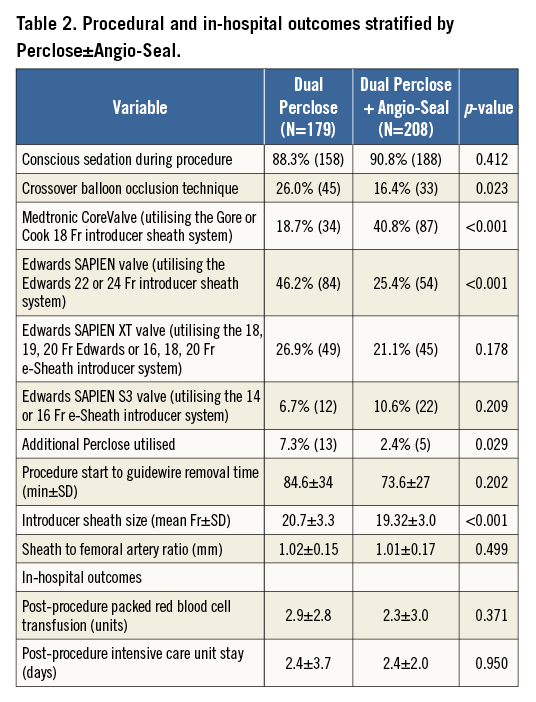
All consecutive patients at the MedStar Washington Hospital Center from May 2007 to January 2015 who underwent a planned transfemoral percutaneous TAVR in addition to the utilisation of at least dual PP devices were included in this study. This selected cohort was further divided into two groups of patients: those who had dual PP vs. dual PP with adjunctive AS in vascular closure. This is a retrospective, observational evaluation of prospectively collected baseline, procedural and post-procedural data stored in our institution’s aortic valve stenosis database. The global TAVR population in our institution comprised 780 patients, up to January 2015. Exclusion criteria for this study included an initial plan for a surgical transfemoral cut-down approach (n=42). The internal institutional review board of the MedStar Washington Hospital Center approved this study. The eligibility of the patients to undergo TAVR after appropriate screening was based on the consensus of our comprehensive Heart Team. The screening process included a baseline comprehensive medical history, physical examination, surgical risk assessment (Society of Thoracic Surgeons [STS] score), frailty index testing, and multiple diagnostic non-invasive and invasive tests deemed necessary for a standard pre-TAVR evaluation. In addition, computed tomography of the cardiac structures and peripheral vasculature for annular and iliofemoral sizing, respectively, were also completed. The procedural parameters also included the calculation of the sheath to femoral artery ratio (SFAR), defined as the ratio between the sheath outer diameter (in millimetres) and the femoral artery minimal luminal diameter (in millimetres) on the setting of a published ratio of ≥1.05 as a cut-off for predicting higher rates of VARC major complications2.
OUTCOMES METHODS AND DEFINITIONS
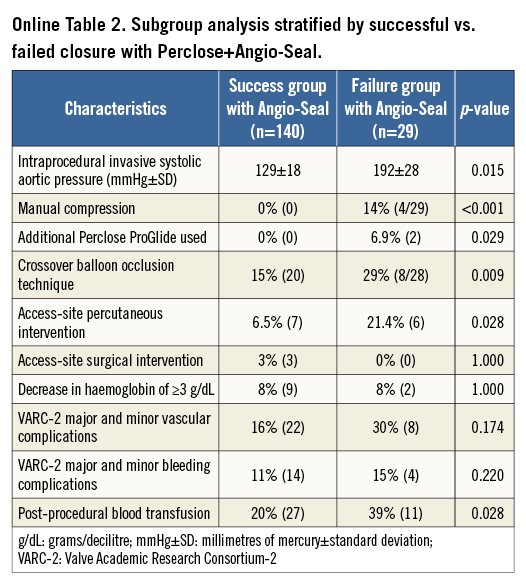
The main objectives of this study were to describe the technique of this novel adjunctive use of AS, to demonstrate its feasibility in the setting of vascular closure in TAVR procedures, and to demonstrate its safety profile with regard to the primary endpoints of in-hospital VARC-2 safety outcomes10 of major and minor vascular complications, VARC-2 life-threatening or disabling bleeding, and VARC-2 major and minor bleeding compared to the default dual PP strategy. Secondary endpoints included in-hospital serious vascular complications requiring percutaneous or surgical interventions, including vascular perforation or dissection, peripheral ischaemia or acute limb ischaemia, arteriovenous fistula, pseudoaneurysm, and access-site haematoma. All clinical events were adjudicated by independent cardiologists who determined the nature of each event. The subgroup analysis included all patients who received dual PP+AS devices and was further divided into two arms: (a) a success group with achieved haemostasis, and (b) the failure group, which required additional interventions, i.e., percutaneous or surgical interventions for vascular closure.
DESCRIPTION OF PROCEDURE
The TAVR procedures were all conducted in the hybrid catheterisation suites of the MedStar Washington Hospital Center. After femoral access was achieved and the dual PP pre-close technique was performed, the TAVR procedure was completed in the conventional manner. Of note, the PP is the default, suture-mediated closure device for TAVR procedures in our catheterisation laboratory. Failure of the dual PP device was defined as the failure to achieve complete haemostasis at the arteriotomy site leading to alternative additional treatments (not including manual compression and not including adjunctive AS in selected patients) to achieve complete haemostasis. The caveat was that, during our TAVR procedures, the adjunctive use of AS was not strictly considered a failure of the dual PP device, as a majority of our patients would be expected to achieve full haemostasis if performance of additional prolonged manual compression were allowed. An overview of the algorithm used at our institution to achieve haemostasis after transfemoral percutaneous TAVR is depicted in Figure 1. Intraprocedural unfractionated heparin was used in all of the study procedures, and protamine use to reverse anticoagulation, if deemed necessary, was left to the discretion of the operator.
Description of technique: successful immediate haemostasis with the dual Perclose ProGlide devices (Figure 1).
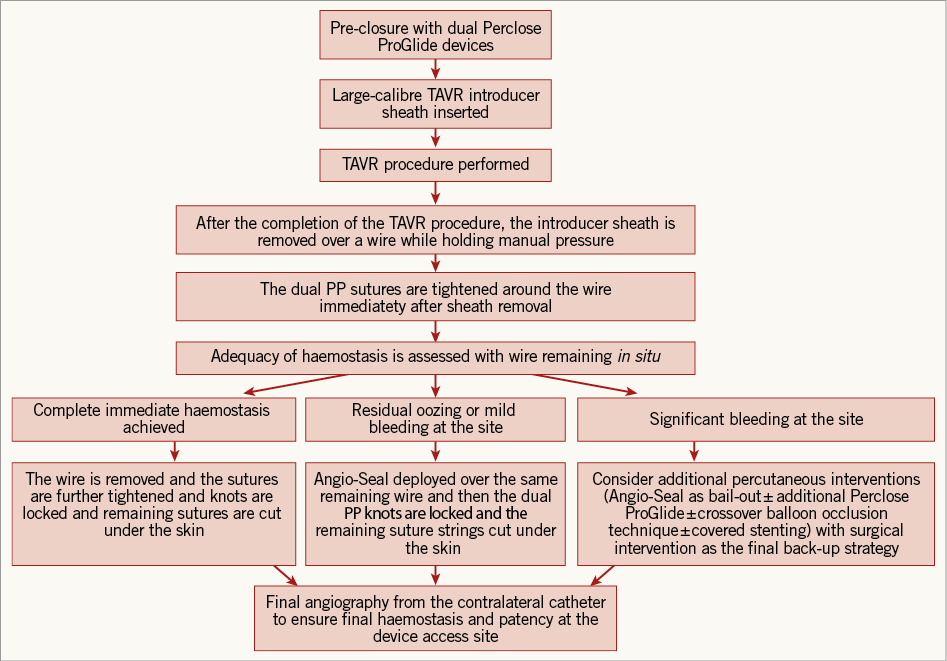
Figure 1. Algorithm of access-site femoral arterial closure in percutaneous TAVR.
The dual PP pre-close deployment technique is performed in the conventional manner8. After the conclusion of the TAVR procedure, the large-bore TAVR delivery sheath is removed and the dual PP sutures are both tightened around the guidewire. At this point, if there is no residual bleeding (i.e., complete immediate haemostasis), then the wire is removed and the dual PPs are further tightened and the knots are locked.
Description of technique: adjunctive Angio-Seal closure device as an alternative technique in selected patients with residual oozing or mild bleeding (Figure 1).
In selected patients who demonstrate signs of oozing or mild bleeding with incomplete immediate haemostasis with the initial dual PP devices, the decision is usually made to insert an AS device (8 Fr calibre), in the conventional manner, to create a final immediate seal of the arteriotomy as an adjunctive measure. The rationale for the augmentation with the AS device in this cohort is twofold: (a) to achieve rapid complete haemostasis at the access site, and (b) to minimise time in the duration of the procedure with accelerated complete haemostasis without the need for prolonged manual compression. In this scenario, the adjunctive AS strategy is used as an alternative to prolonged manual compression in vascular closure when the dual PP fails to achieve immediate complete haemostasis.
Description of technique: additional manoeuvres in the event that the closure device fails to achieve haemostasis with residual significant bleeding (Figure 1).
In the event that the dual PP devices fail to achieve haemostasis with signs of residual significant bleeding, the subsequent step is usually an attempt to use an adjunct AS in a bail-out strategy by initially inserting the AS sheath as a “test,” and assessing the degree of bleeding from around the sheath. If the bleeding is significantly controlled with the AS sheath “test”, then the AS device will subsequently be deployed. If the “test” fails to control the bleeding signifying probable failure with an AS device, then usually a third PP will be used and subsequently the “test” step with the AS sheath will be repeated. If these mentioned manoeuvres are unsuccessful in achieving haemostasis, then a peripheral angiography to visualise and assess the arteriotomy site further will be performed, and the usual subsequent step will be to perform an adequate crossover balloon occlusion technique (CBOT). Thereafter, if haemostasis is still not achieved, the decision is made to pursue a contralateral percutaneous covered stent placement. Of note, in the event of severe bleeding after the dual PP sutures are tightened, the usual subsequent step is to re-insert the large-bore TAVR delivery sheath to avoid excessive bleeding and pursue a contralateral percutaneous covered stent placement. The final back-up strategy to achieve haemostasis is surgical intervention.
Statistical analysis
The baseline characteristics, procedural data, and in-hospital outcomes were evaluated. The analysis for comparison of the groups was performed by using SAS version 9.1 (SAS, Cary, NC, USA). Continuous variables were expressed as mean±SD for normally distributed variables. Categorical variables were expressed as percentages (%). The Student’s t-test was used to compare means of continuous variables and the χ2 test or Fisher’s exact test was used to compare categorical variables. A p-value of <0.05 was considered statistically significant.
Results
Overall, a total of 387 consecutive patients (50% of the total TAVR population) were evaluated. Furthermore, the groups that were compared were patients who had dual PP (n=179) vs. dual PP+AS device (n=208). Baseline patient clinical and imaging characteristics are demonstrated in Table 1, and Online Table 1, respectively. The mean age of the patients was 83 years, and 55% of patients were male. Among the pertinent baseline imaging characteristics (Online Table 1), there were no significant differences in the degree of moderate or severe iliofemoral calcification, tortuosity, or calibre of the common femoral artery between the two study groups. With regard to the pertinent procedural outcomes (Table 2), there was a higher rate of CBOT utilised for the dual PP vs. the dual PP+AS group (26% vs. 16.4%, p=0.023). The SFAR (Table 2) were similar (1.02 mm vs. 1.01 mm, p=0.499) in the PP vs. PP+AS groups, indicating that this is less than a predicted high-risk cohort for VARC major vascular complications. There were no statistically significant differences between the dual PP vs. dual PP+AS groups with regard to the primary endpoints of in-hospital VARC-2 major vascular complications (8.0% vs. 6.6%, p=0.592), minor vascular complications (18.4% vs. 13.7%, p=0.218), life-threatening or disabling bleeding (5.1% vs. 3.0%, p=0.291), major bleeding (1.7% vs. 1.5%, p=1.000), and minor bleeding (14.4% vs. 10.6%, p=0.271) (Figure 2). In addition, the rates of the secondary endpoints of serious vascular complications requiring percutaneous or surgical interventions were also similar between the two groups (Figure 2).
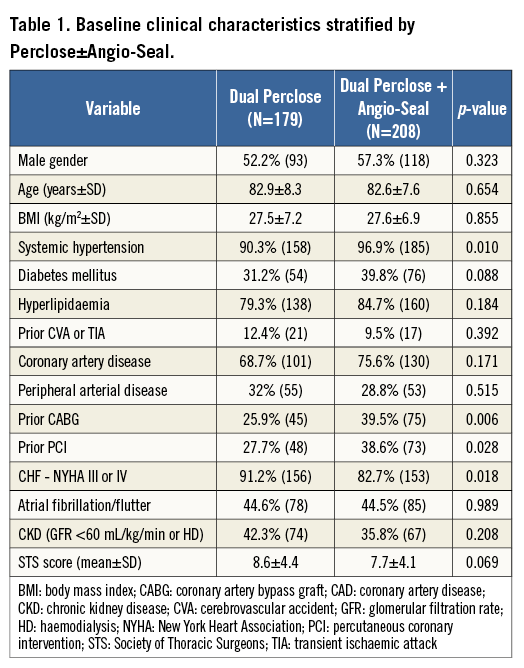
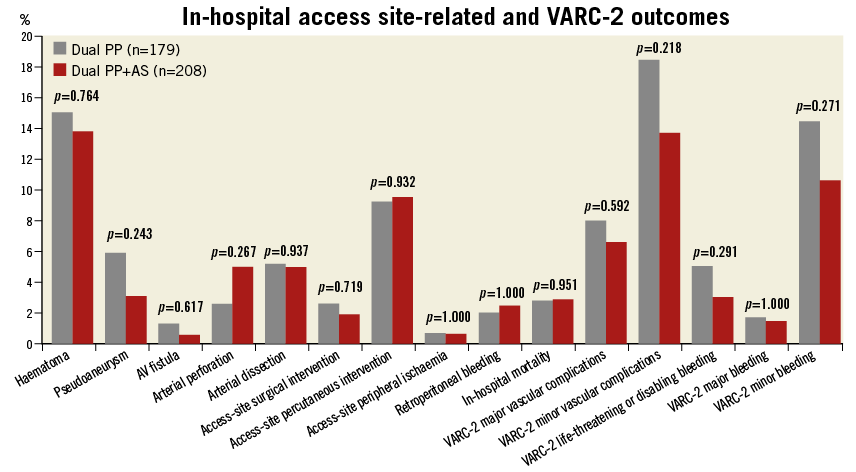
Figure 2. In-hospital access site-related serious vascular complications and VARC-2 safety outcomes.
The subgroup analysis included 169 patients within the dual PP+AS group who had either full haemostasis (n=140) or failure thereof (n=29) (Online Table 2). In the failure group, the procedural characteristics demonstrated statistically significant higher rates of the following parameters: intraprocedural hypertension, manual compression, additional closure device used, transfemoral CBOT, and access-site percutaneous intervention (Table 2). However, there was no significant difference in the rates of access-site surgical interventions. The in-hospital clinical safety endpoints demonstrated no differences in VARC-2 major or minor vascular access-site complications, or VARC-2 major or minor bleeding complications (Online Table 2).
Discussion
The main results of this study demonstrate that the rates of in-hospital VARC-2 vascular safety outcomes between the PP and PP+AS groups were similar. The main conclusion is that the adjunctive use of the AS closure device to augment the dual PP pre-close approach is feasible, efficacious, and safe in percutaneous femoral closure, and may be considered as a bail-out or an alternative strategy when the dual PP closure technique fails to obtain complete immediate haemostasis. Although this study does not provide any objective evidence regarding the additional benefit of reducing the time to patient arousal or the improvement of the general efficiency in the cathlab with this new approach, there is subjective, centre-wide accepted evidence that the above advantages hold true when the adjunctive use of AS is utilised. On the other hand, in contrast to the immediate haemostasis achieved with the successful adjunctive utilisation of AS in the majority of cases, the alternative would be to utilise prolonged manual compression. Otherwise, since the start of the routine adjunctive AS use for TAVR procedures in our catheterisation laboratory in June 2012, the protocol has been that, if there is no significant bleeding with only residual oozing/mild bleeding as evident in the majority of the percutaneous TAVR procedures, then the direct AS augmentation is used for immediate haemostasis without the prolonged time needed with the alternative utilisation of manual compression and/or CBOT.
With regard to the subgroup analysis of the successful vs. failed dual PP+AS groups, importantly it is demonstrated that, even if the adjunctive use of Angio-Seal fails, subsequent haemostasis is achieved with conventional techniques without an increased incidence of surgical interventions.
The utilisation of this adjunctive AS technique in the setting of large-bore post-TAVR vascular closure has, to our knowledge, not been previously described in the literature. There have been reports in the literature of successful utilisation of a third or fourth PP device to control haemostasis if the initial dual PP have failed11 in the setting of endovascular aneurysmal repair. However, our rationale to use the AS device as an adjunct after the PP is that the dual mechanism of the AS, which includes providing the fine adjoining approximation with its collagen plug and creating a footplate abutting under the PP sutures in addition to the pro-coagulant properties of the collagen plug, certainly contributes to the augmentation of haemostasis. In addition, there have been reports of using the AS device off-label to close up to 9-12 Fr arteriotomy sites in the contexts of balloon aortic valvuloplasty and endovascular aneurysm repair procedures, and it has proven to be safe and feasible in these settings as well12,13.
In the literature, the failure of the PP closure device requiring percutaneous intervention or surgery or leading to related access site-related bleeding has been reported to be around 4-9% of cases, with sheath sizes ranging from 18 to 24 Fr in most of the published studies1,5-7,9. We have not specifically assessed the PP failure rates with TAVR in this study given that, in this case series, even if there was residual oozing or mild bleeding from the access site with the dual PP, an adjunctive AS device was still deployed to achieve immediate complete haemostasis, reduce time to arousal of the patient, and reduce cathlab turnaround time to aid in overall efficiency. Hence, the main objective of this evaluation was to describe the technique and assess the safety of the adjunctive AS device to augment the haemostasis further.
Study limitations
This is a retrospective study; therefore, it has significant bias inherent in its design. There is a possibility that multiple confounding factors exist, including different operator preference, patient anatomy, patient coagulopathy status, variation in protamine use, variation in the checking of the activated clotting time, and targets during the procedure. The other limitation is that it is possible that many cases of residual bleeding after the PP tightening may have resolved with simple prolonged manual compression, although at our large centre one of our aims is generally to reduce the procedure time and increase cathlab efficiency.
Conclusions
In conclusion, the technique of augmenting the dual PP with an adjunct AS is feasible, efficacious, and safe for percutaneous femoral closure in selected patients undergoing TAVR, and may be considered as a bail-out or an alternative strategy when the dual PP closure technique fails to obtain complete haemostasis. This series highlights the frequent clinical need to use adjunctive measures in addition to suture-mediated closure devices in TAVR procedures.
| Impact on daily practice This is a useful, modified vascular closure technique in transfemoral percutaneous TAVR, which utilises the AS device as an adjunctive measure to augment the dual PP technique to aid in haemostasis in selected cases. This technique is feasible and safe, and may be considered as a bail-out or an alternative strategy when the dual PP closure technique fails to obtain complete haemostasis. |
Conflict of interest statement
R. Waksman reports being a consultant to Abbott Vascular, Biotronik, Boston Scientific, Medtronic, and St. Jude Medical, being on the speakers bureau of AstraZeneca, Boston Scientific, and Merck, and receiving grant support from AstraZeneca, Biotronik, and Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data