
Introduction
Utilisation of transcatheter aortic valve replacement (TAVR) is increasing. Vascular complications after transfemoral TAVR are still of concern. The majority are related to the large bore puncture site1. Currently, large bore arteriotomy closure relies on suture-based techniques, such as the Perclose ProGlide® or Prostar® XL devices (Abbott Vascular, Santa Clara, CA, USA). Closure devices such as MANTA® (Teleflex, Wayne, PA, USA) or PerQSeal® (Vivasure Medical, Galway, Ireland) are designed to seal large bore arteriotomies and have recently become available. The novel InClosure® vascular closure device (VCD) (InSeal Medical, Caesarea, Israel) provides haemostasis using a flexible intravascular biodegradable membrane supported by a nitinol frame (Figure 1). We report our single-centre experience using this novel VCD.
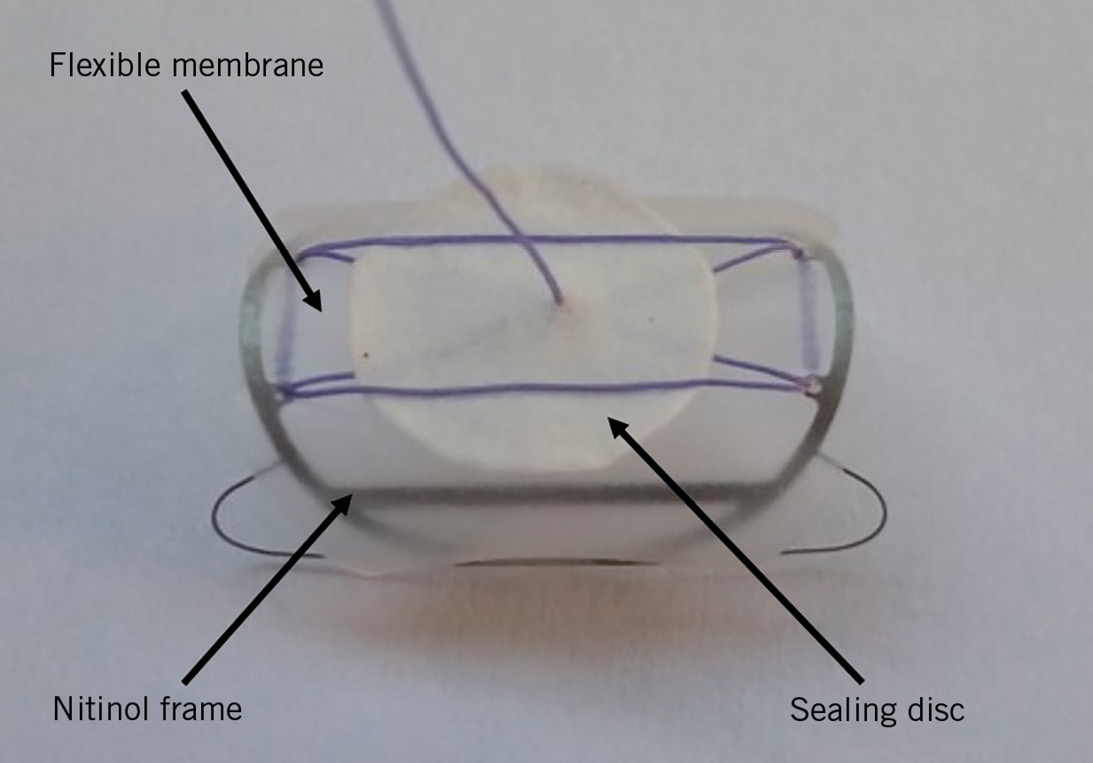
Figure 1. The InClosure VCD.
Methods
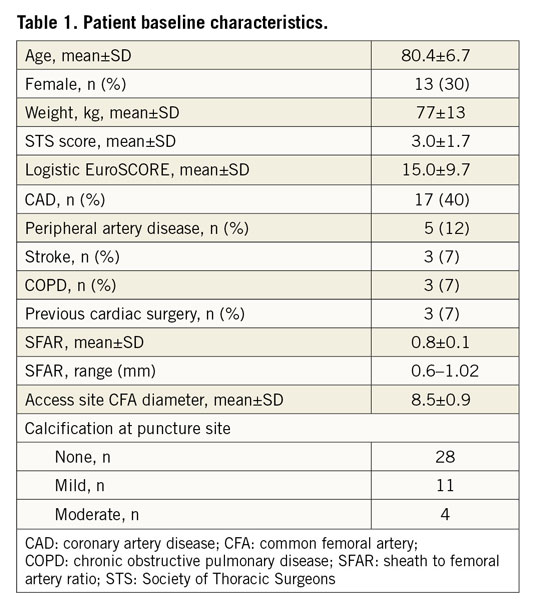
Between June 2018 and March 2019, 43 patients were included in a retrospective investigation at the Department of Cardiovascular Surgery at the German Heart Center, Munich. Patients were not preselected, but VCD utilisation was dependent on InSeal representatives being at the site providing VCDs. A common femoral artery (CFA) diameter larger than 6 mm was mandatory for the use of the InClosure device. Periprocedural data were retrospectively analysed from the prospectively maintained AVIATOR-TAVI registry (www.clinicaltrials.gov: NCT01390675). Closure of the large bore puncture site was achieved by InClosure VCD implantation at the end of the TAVR procedure. Access site-related vascular and bleeding complications were recorded according to the Valve Academic Research Consortium (VARC)-2 recommendations.
DEVICE DESCRIPTION AND IMPLANTATION TECHNIQUE
The InClosure VCD (Figure 1) consists of a biodegradable membrane supported by a nitinol frame. It is implanted across the puncture site, while its 20 mm length is designed to ensure full coverage of the puncture hole. The nitinol frame expands the VCD, promoting the coupling of the membrane to the vessel wall. The flexibility of the membrane is intended to compensate for irregularities of the arterial wall. In addition, the InClosure VCD includes a smaller sealing disc, made from the same biodegradable polymer as the main membrane. This disc is not connected to the frame and is intended to serve as an additional sealing layer independent of the main membrane. The device has a profile of about 0.1 mm. There are no components of the VCD which are extravascular, other than a 4.0 polyglycolic tether wire. Intraoperatively, the location of the puncture site and the distance to the femoral bifurcation of at least 1.5 cm are confirmed by selective angiography through a 6 Fr sheath after puncture of the CFA.
After transcatheter valve implantation, the large bore sheath is retracted to approximately 5 cm above the puncture site. The InClosure delivery system (Figure 2) is then advanced into the sheath. Fully advanced, the VCD aligns with the distal tip of the sheath. After release of the safety knob, the handle of the delivery system is firmly fixed and the TAVR sheath is pulled back into the handle up to a hard stop. A marker at the handle indicates the exposure of the crimped VCD. While pulling back the whole system, the distal end of the VCD crosses the puncture site into the distal CFA. Thus, the VCD is pushed against the anterior vessel wall. Thereby, a release mechanism is activated, expanding the nitinol frame and coupling the membrane to the ventral wall of the artery sealing the puncture hole. The device had a CE mark (no. 2409) at the time of the first implantation.
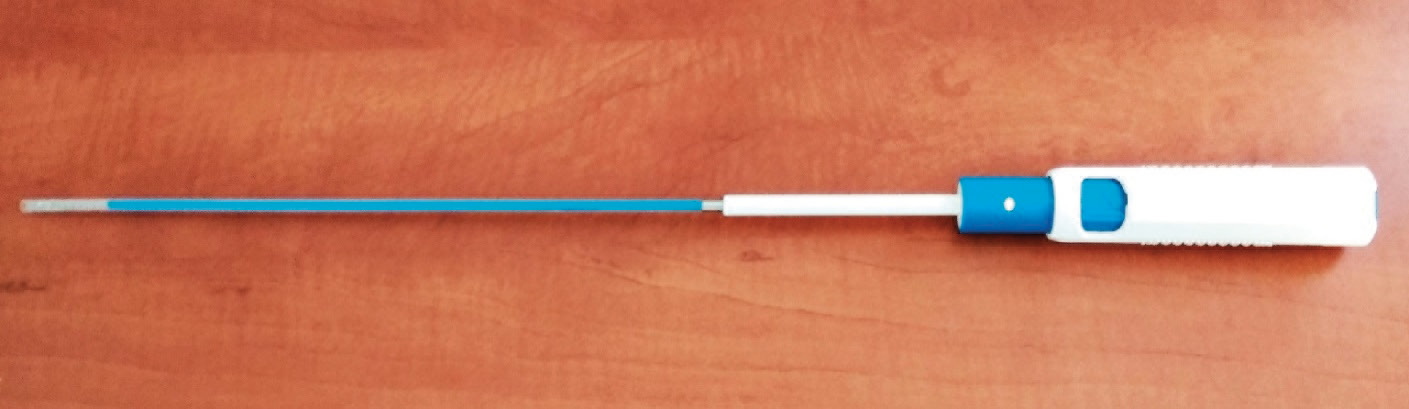
Figure 2. The InClosure system with the preloaded VCD at the tip of the catheter.
Results
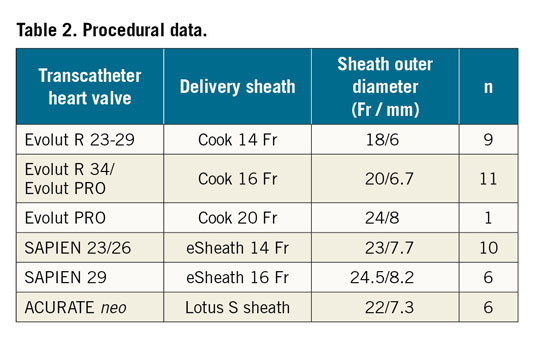
In 43 patients undergoing transfemoral TAVR, the large bore puncture hole was sealed by implantation of the InClosure VCD (Moving image 1). Table 1 summarises the baseline demographics. Procedural characteristics and results are shown in Table 2 and Table 3. The sheath outer diameter was ≥20 Fr in 79% of the cases and >20 Fr in 53% of the cases. Successful device implantation leading to complete haemostasis was achieved in 42 (98%) of the 43 patients. In one patient, an initially unrecognised iatrogenic dissection of the femoral artery caused by the primary vessel puncture and insertion of a standard wire prevented adequate device positioning, leading to a VCD failure. In two patients, full haemostasis was achieved by additional percutaneous transluminal angioplasty (PTA) balloon dilatation of the device.
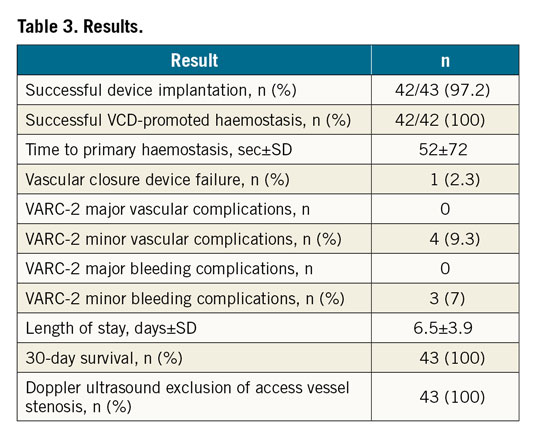
No VARC-2 major vascular complication was recorded. Three pseudoaneurysms and one haematoma accounted for the four minor vascular complications at the large bore access site (9.3%).
No VARC-2 major bleeding complication was recorded. Three minor bleeding complications occurred (7%).
Thirty-day survival was 100% without additional bleeding or vascular adverse events at 30 days.
Discussion
To the best of the authors’ knowledge, these are the first clinical results after CE-mark approval of the novel InClosure VCD. Reliable implantation of the VCD was achieved with successful haemostasis at the large bore puncture site. No major VARC-2 vascular complication was recorded.
A considerable range of major vascular complications has been reported for different VCDs, with 1.9% to 20% for the ProGlide2,3, 0.4% to 7.4% for the Prostar2,4 and 2.3% to 10% for the MANTA3,4. Even these inconsistent data demonstrate the need for further improvements in vascular closure after transfemoral TAVR.
No VARC-2 major bleeding complication occurred in our study. The rates of minor vascular (9.3%) or bleeding (7%) complications were low and are comparable with rates reported for established closure devices2,3,4. A larger patient cohort is needed to verify our initial results, and randomised trials are needed to demonstrate the benefit of this new device. Nonetheless, we achieved high device success with a low complication rate using the novel InClosure VCD.
Limitations
A limitation of this study is the small patient cohort. A potential selection bias and the absence of an independent adjudication committee may have influenced the reported clinical results. Larger patient numbers and randomisation with other VCD systems must confirm the initial findings.
Conclusion
The novel InClosure VCD seals large bore puncture holes promoted by an intravascular biodegradable membrane supported by a nitinol frame. It is compatible with all commonly used TAVR sheaths. In our initial single-centre experience, VCD implantation was reliable with a high device success rate after percutaneous transfemoral TAVR. No major vascular or bleeding complications were noted. The InClosure VCD might be effective in decreasing vascular complication rates in TAVR.
|
Impact on daily practice Vascular complications are still of concern after TAVR. The InClosure VCD is a dedicated device sealing large bore puncture holes after transfemoral TAVR with low complication rates in an initial patient cohort. Randomised trials should compare the new device to other available VCDs. |
Conflict of interest statement
H. Ruge reports proctor honoraria from InSeal Medical, during the conduct of the study. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Implantation of the InClosure VCD at a large bore puncture site after transfemoral TAVR.

