Despite a continuous decline in procedure-related complications during transcatheter aortic valve implantation (TAVI) procedures over the last decade, vascular complications represent a persisting challenge even in contemporary, lower-risk patients1. From a conceptual standpoint, two main strategies have been pursued to achieve safe and effective large-bore arteriotomy closure: i) a percutaneous dual suture-based closure technique, including subsequent modifications of placement technique2; and ii) use of plug-based vascular closure devices (VCD) specifically designed for large-bore arterial access closure. Recently, the randomised MASH3 and CHOICE-CLOSURE4 trials have reported clinical outcomes following different vascular access closure strategies in patients undergoing TAVI. A pure plug-based vascular closure technique using the MANTA VCD (Teleflex) was associated with numerically higher rates of access site-related vascular complications compared to a primary suture-based strategy using the double ProGlide (Abbott) technique in the MASH trial and with a significantly higher rate of access site- or access-related vascular complications in the larger CHOICE-CLOSURE trial34. In the latter trial, major access site-related vascular complications were observed in <4% of patients, indicating that the primary endpoint was mainly driven by minor vascular complications, such as access site haematoma or a pseudoÂaneurysm4. Overall, randomised data did not replicate the encouraging results of initial observational studies regarding the use of the MANTA VCD56.
In this issue of EuroIntervention, Dumpies et al report the efficacy and safety of a bailout use of the MANTA VCD following a failed suture-based closure during TAVI in a high-volume German TAVI centre. ProGlide VCD failure was observed in 4.3% (107/2,505) of the entire cohort − with 83% of failures occurring after sheath removal − and 61.7% (66/107) of these patients underwent bailout treatment with the MANTA VCD. The bailout strategy was effective in achieving haemostasis at the access site without the need for additional treatment in three-quarters of the cases (75.8%). Moreover, safety was maintained with this technique in the large majority of cases (86.4%), which indicated freedom from vascular dissection, stenosis or occlusion requiring intervention. Importantly, moderate (33.3%) or severe (31.8%) calcification of the iliofemoral vessels was present in almost two-thirds of patients, and anterior access site calcification was present in almost half (45.5%)7.
The authors should be congratulated for adding further evidence to a rapidly evolving field that has the potential to significantly widen the treatment options and to reduce the need for stent implantation or surgical repair in patients undergoing TAVI procedures. Multivariable analysis showed operator experience to be highly predictive (odds ratio [OR] 12.29) of an effective bailout treatment, whereas the application of more than two ProGlides was shown to be associated with a reduced efficacy (OR 0.02). This latter finding promotes the timely application of a bailout MANTA VCD strategy, before additional vascular manipulation potentially aggravates arterial injury and limits the chance of a successful percutaneous sealing of the arteriotomy site. On the other hand, MANTA VCD implantation as a bailout strategy is technically more demanding compared to its elective counterpart, primarily because of challenging depth measurements in the setting of persistent significant bleeding through a large-bore access, and experience with the technique appears to be of paramount importance. Indeed, a comparison of the efficacy and safety of such a plug-based strategy between the present patient population and the “MANTA cohort” of the CHOICE-CLOSURE trial confirmed both efficacy (75.8% vs 93.4%; p<0.001) and safety (86.4% vs 94.6%; p=0.040) to be significantly lower in the bailout population. Nevertheless, bailout implantation of a MANTA VCD was effective in achieving haemostasis in three-quarters of the patients in a safe manner, thereby drastically reducing the need for covered stent implantation or surgical repair7.
Finally, in addition to the “isolated” suture-based and plug-based techniques, upfront combinations of a suture-based and a small plug-based device have become increasingly accessible in the clinical routine, and initial results from observational studies appear encouraging8. In the pursuit of the safest and most effective VCD strategy, the results from the ongoing randomised ACCESS-TAVI trial (ClinicalTrials.gov: NCT05503199) will surely inform our decision-making in this specific field (Figure 1).
In this prospective, randomised, multicentre, industry-independent trial, two VCD strategies for large-bore vascular access closure following transfemoral TAVI are being compared in a large cohort of 450 patients. The hypothesis which is being tested is that a combined VCD strategy using a combination of one Perclose ProGlide or ProStyle (both Abbott) and one Angio-Seal (Terumo) is superior to a pure suture-based VCD strategy with two ProGlides or ProStyles with regard to the primary endpoint, which is a combination of major or minor access site-related vascular complications or need for additional interventional or surgical procedures related to vascular haemostasis according to Valve Academic Research Consortium-3 criteria during hospitalisation. Although the suture-based double ProGlide VCD strategy has been the predominant option for almost a decade, the aforementioned randomised trials demonstrated a frequent need for an additional small plug-based VCD to achieve complete haemostasis in almost 95% of patients in the CHOICE-CLOSURE trial4.
Such combined efforts, in both identifying the optimal upfront selection of closure devices for large-bore arteriotomy as well as standardising and personalising bailout techniques and workflows for the increasingly rare occurrence of closure system failures, make one look confidently towards the future of TAVI procedures.
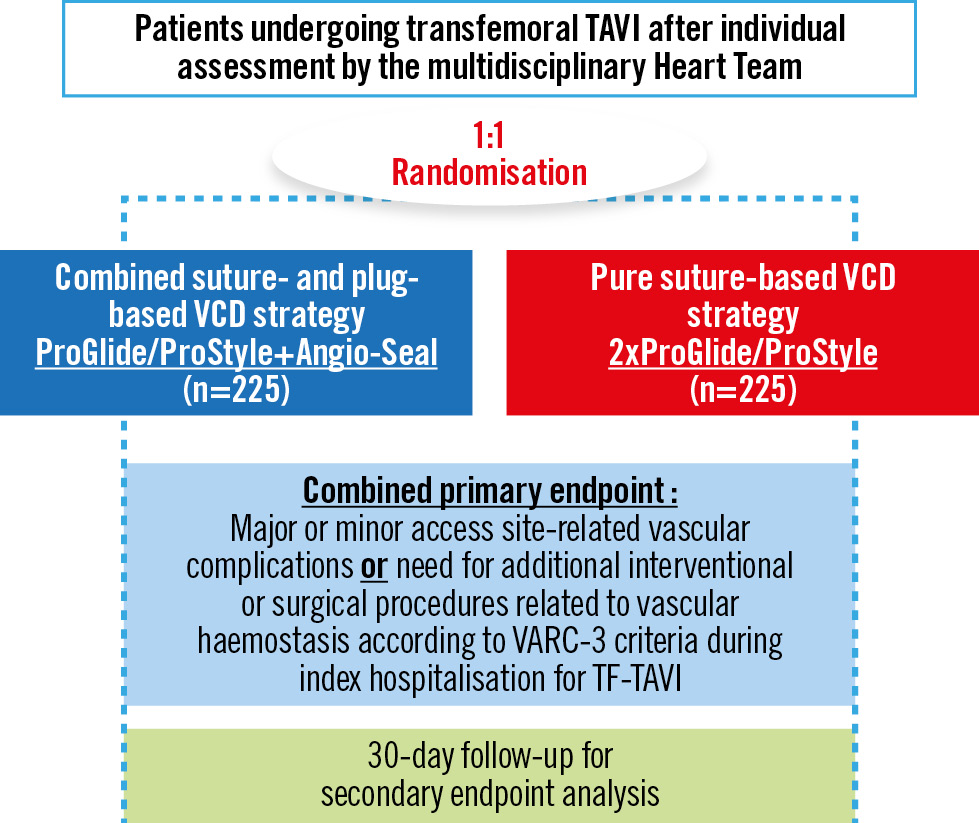
Figure 1. Study flowchart of the randomised ACCESS-TAVI trial. TAVI: transcatheter aortic valve implantation; TF: transfemoral; VARC: Valve Academic Research Consortium; VCD: vascular closure device
Conflict of interest statement
T. Rheude has received lecture fees from AstraZeneca, Abbott, SIS Medical, and Translumina; and a travel grant to the institution from Boston Scientific, not related to this work. E. Xhepa reports lecture fees and honoraria from AstraZeneca, Boston Scientific, and SIS Medical not related to the current work; proctor fees from Abbott Vascular; and financial support for attending meetings and/or travel expenses from Abbott Vascular and SIS Medical.

