Abstract
Aims: To demonstrate the feasibility and efficacy of the novel InSeal VCD for the closure of large puncture holes following percutaneous structural interventions.
Methods and results: Prospective, non-randomised, single-arm, single-centre study with a series of patients submitted to endovascular treatment of abdominal and thoracic aortic aneurysm as well as transcatheter aortic valve implantation in whom the InSeal VCD was used to close the access site. These patients were followed up for one year with clinical examination, ankle-brachial index and Doppler ultrasound. The primary endpoint was the occurrence of major vascular complications at the puncture site. From a total of nine patients screened, seven were selected to receive the InSeal VCD. Technical and therapeutic successes were achieved in all cases. The sheath profiles used in these procedures ranged from 18 Fr to 25 Fr. No major vascular complications were observed during the follow-up period. Average ankle-brachial index pre-intervention and at one-month follow-up were 0.85 and 0.82, respectively.
Conclusions: The InSeal VCD was shown to be effective in achieving acute and chronic haemostasis after usage of higher profile endovascular devices in this study. These results translated into no clinical complications up to one-year clinical follow-up.
Introduction
Percutaneous endovascular procedures offer an efficacious and less invasive approach to treat structural and acquired cardiovascular pathologies and are therefore becoming progressively more prevalent.
However, these procedures are not free of complications, mostly related to the access site. The relatively high profile of the devices used during such interventions usually requires surgical dissection and suture, which might result in local pain and higher risk of bleeding, haematomas, pseudoaneurysm, infection and other serious complications, which can occur in 9-17% of cases1-5.
Vascular closure devices (VCD) are an attractive option to overcome these complications but their use is generally limited to small puncture bores (6-10 Fr) while some endovascular procedures (repair of aortic aneurysm, transcatheter aortic valve replacement, etc.) require devices of a much higher profile (18-25 Fr)6.
The only currently available VCD for bigger puncture bores is the Prostar® XL/Perclose ProGlide® (Abbott Vascular, Santa Clara, CA, USA), which can be used for 5 Fr to 21 Fr orifice closure. However, the use of this device is complex, necessitates a learning curve, requires preprocedural preparation, might cause vessel deformation, and is not infrequently related to local pain and bleeding. Furthermore, haemostasis is obtained only in about 85-90%2-5, and balloon inflation, at the closure site, might be necessary to stretch the Prostar sutures and to allow contraction of the puncture and thrombus formation that seals the closure leaks.
The novel InSeal VCD (InSeal Medical Ltd., Caesarea, Israel) is based on an internal biodegradable membrane, which seals the puncture. The membrane is supported against the vessel wall by a specially designed self-expandable frame, and is delivered into the vessel, in a collapsed configuration, via a sheath. This device was conceived to close puncture holes up to 25 Fr. It does not require preprocedural preparation and it permits re-access through the same vascular site within 26 weeks. We sought to demonstrate the feasibility and efficacy of this novel VCD for the closure of large puncture holes (18 Fr to 25 Fr) following percutaneous structural interventions.
Methods
STUDY DESIGN AND POPULATION
The study was a prospective, non-randomised, single-arm, single-centre study with a series of patients submitted to endovascular treatment of abdominal and thoracic aortic aneurysm as well as transcatheter aortic valve implantation, in whom the InSeal VCD was used to close the access site.
Patients were eligible for the study if they were ≥18 years and their femoral artery measured between 6 and 10 mm. Exclusion criteria comprised severe calcification/tortuosity at the puncture site or the puncture site being less than 12 mm from the femoral bifurcation. Additionally, patients were excluded if they had any branch ≥2.5 mm within 4 cm proximal to the puncture site.
In the present manuscript we report the technical success of the device, as well as the therapeutic success of the intervention and the frequency and type of puncture-site-related complications observed during the procedure and in the one-month and one-year follow-up periods. Vascular Doppler was performed at different time points, typically 24 hours, one month, six months and one year post-procedure.
The local ethics committee approved the study and all patients signed informed consent prior to the procedure.
DEVICE DESCRIPTION AND DEPLOYMENT TECHNIQUE
The InSeal VCD comprises three main components (Figure 1): a) a self-expanding nitinol frame with a length of 22 mm and a thickness of 100 μm; b) an internal L-lactide/caprolactone (PLC) biodegradable membrane supported by the nitinol frame; and c) a bioresorbable polyglycolic acid (PGA) tether.
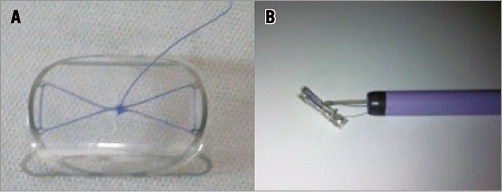
Figure 1. A) The InSeal VCD device fully opened. B) The InSeal VCD device at the exit of an 18 Fr introducer.
Once the endovascular procedure is completed, and prior to InSeal VCD placement, an occlusion balloon is inflated proximal to the puncture site as a precautionary safety measure so that the blood flow may be stopped in the case of a failed closure procedure. The occlusion balloon is not an essential part of the InSeal procedure and may be replaced by distal compression, for stopping blood flow, during device delivery and deployment. Next, the InSeal VCD is introduced into the sheath until the VCD is positioned at the tip of the sheath. The sheath is then pulled back, exposing the VCD within the vessel about 3 cm distal to the puncture site (Figure 2). The VCD delivery system is then pulled back until some resistance is felt, which means that the folded InSeal VCD is positioned across the puncture site. Next, the release wire is pulled to deploy the VCD, allowing the nitinol frame to expand and to couple the membrane against the vessel wall. At this point the biodegradable PGA tether is fixed to the patient’s skin, using a sterile bandage (Steri-Strip) keeping the tether under a minimum traction for about 12 hours. Alternatively, the tether wire may be secured in the skin sutures used to close the skin opening.
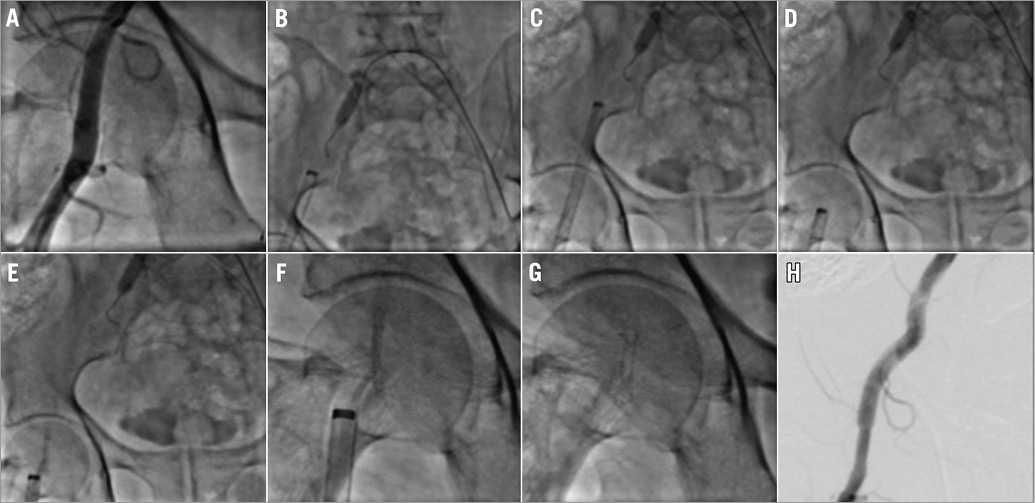
Figure 2. Step-by-step InSeal VCD device implantation. A) Prior arteriogram demonstrating a suitable artery for device deployment. B) Positioning balloon occluder upstream. C) Introduction of the InSeal VCD device in an 18 Fr introducer. D) Pullback of the introducer. E) Deployment of the device. F) View at higher magnifications. G) Pullback of the introducer device and accommodation in the arterial wall. H) Final arteriogram.
Ambulation is allowed within six to eight hours of the procedure; however, this is not always possible due to the nature of the main procedure to which the patients were submitted. Moving image 1 illustrates a procedure with this device.
STUDY ENDPOINTS AND DEFINITIONS
The primary endpoint of this first-in-man evaluation was to demonstrate the feasibility of this novel closure device as well as to report the occurrence of major vascular complications at the puncture site. Major complications were defined as the need for a blood transfusion, the need for surgical intervention and the occurrence of thromboembolic complications with clinical repercussion (e.g., frailty, cyanosis, pain, etc.) related to the limb treated with the InSeal device.
Technical success was defined by the ability to position, deploy and fix the InSeal VCD. Therapeutic success was defined as the occurrence of technical success with no major complication during the in-hospital period.
Results
A total of nine patients were initially screened for the study but two were excluded due to adverse puncture site characteristics. Among the treated patients, most were male (71.8%). The most frequent comorbidities were hypertension (85.7%) and peripheral vascular disease (71.8%) (Table 1). Mean hospital stay ranged from 2 to 29 days (mean of 10.3 days). The sheath profile used in these procedures ranged from 18 Fr to 25 Fr. Table 2 briefly describes the main characteristics of each enrolled patient as well as their outcomes.
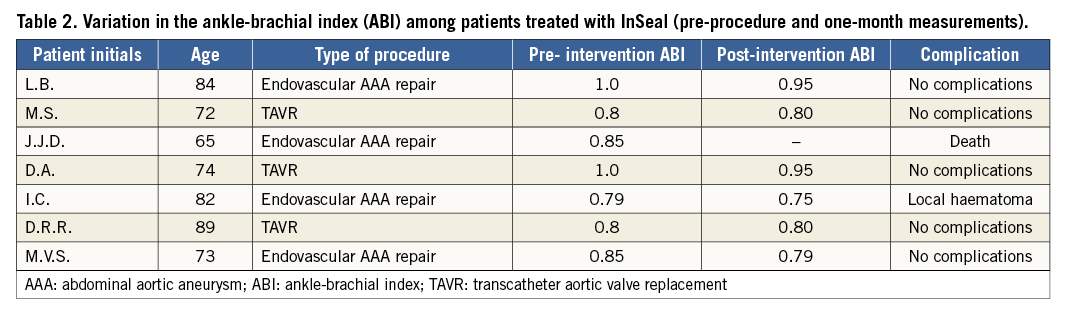
Technical and therapeutic success was achieved in all cases. Additionally, no complications were observed during the follow-up period. Average ankle-brachial index pre-intervention and at one-month follow-up was 0.85 and 0.82, respectively.
In one case, a local haematoma was noticed, but it did not require any additional intervention. In the late follow-up period there was one, non-procedure-related death.
Figure 3 illustrates the Doppler imaging obtained immediately after InSeal VCD deployment and at one-year follow-up, after the device had been fully resorbed.
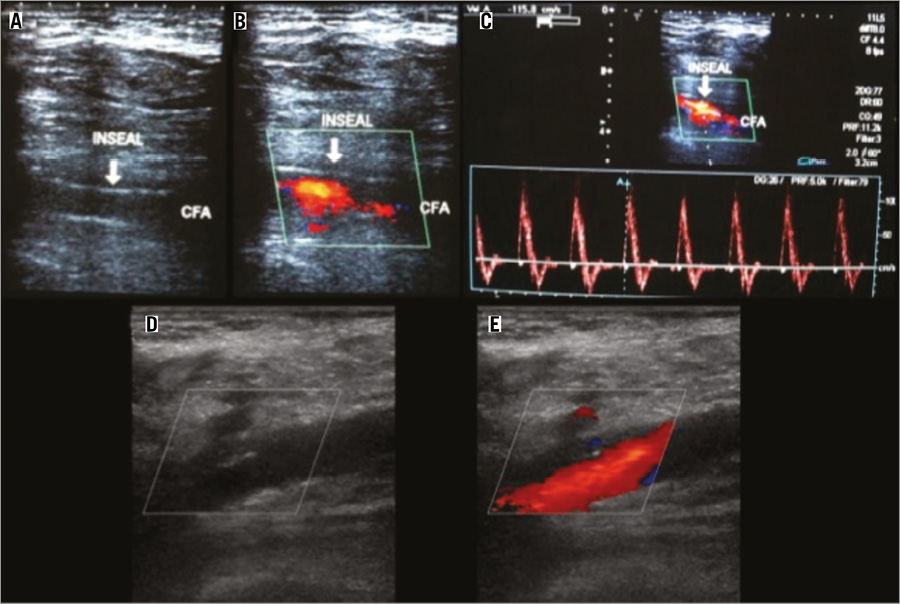
Figure 3. InSeal VCD deployment. A) B-mode vascular ultrasound demonstrating the device fit in arterial wall one month after implantation. B) Colour mode vascular ultrasound with no endoleak. C) Spectral mode demonstrating triphasic flow in the common femoral artery. D) & E) One-year Doppler follow-up of InSeal VCD implantation..
Discussion
The present study demonstrated the feasibility of the InSeal VCD for the closure of large puncture holes. The lack of major vascular complications, despite the high profile of the endovascular devices used, suggests the efficacy of this novel device in preventing complications in a scenario where most vascular closure devices have been proven to be ineffective or difficult to use.
The endovascular approach to cardiovascular and structural diseases is associated with puncture-site-related complications in 1 to 9%1-7 of cases. The risk increases with the use of higher profile devices, prolonged procedure time, and number of previous interventions at the same vascular site, as well as the use of concomitant antiplatelet and anticoagulant therapy1-7.
The first percutaneous closure device for femoral access was initially tested in 1999 by Hass et al. Since then, a broad array of different devices has been tested with technical success rates ranging from 62% to 100%1-11. This wide variance in success rate is related to the punctured vessel characteristics and the sheath diameters used for the procedures. Higher success rates are more often noticed among lower profile sheaths (6 Fr to 12 Fr)9,12-14.
Since anatomical difficulties pose most of the problems to the success of vascular closure devices, a careful evaluation of the target vessel and the choice of an ideal puncture site are the key points for increasing the efficacy and safety of these devices. Among the vessel characteristics, the presence and amount of calcification at the puncture site and in the nearby region are often related to difficulty in the use of VCD and also to vascular complications. Patients with severe calcifications were excluded from the present study.
Some ischaemic complications related to the puncture site, such as intermittent claudication, might only be noticed at late follow-up, after hospital discharge. Such chronic complications may, at least partially, originate from vessel deformation and stenosis caused by vessel suturing. Of note, none of the treated patients with the InSeal VCD presented such a complaint. Also, vascular Doppler performed at one month and one year confirmed the full patency of the punctured artery with no flow disturbance at the site of the InSeal deployment.
Conclusions
In the present study, the InSeal VCD was shown to be feasible in achieving acute and chronic haemostasis after usage of higher profile endovascular devices. These results translated into no clinical complications up to one year of clinical follow-up.
Larger, prospective and randomised studies should confirm these promising preliminary results.
Funding
Instituto Dante Pazzanese has received a research grant from InSeal Medical to conduct the study.
Conflict of interest statement
A. Penner serves as a company employee and is a stockholder of InSeal Medical. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. The InSeal procedure.

