
Abstract
Catheter-based interventions have become a less invasive alternative to conventional surgical techniques for a wide array of cardiovascular diseases but often create large arteriotomies. A completely percutaneous technique is attractive as it may reduce the overall complication rate and procedure time. Currently, large bore arteriotomy closure relies on suture-based techniques. Access-site complications are not uncommon and often seem related to closure device failure. The MANTA VCD is a novel collagen-based closure device that specifically targets arteriotomies between 10 and 22 Fr. This technical report discusses the MANTA design concept, practical instructions for use and preliminary clinical experience.
Introduction
For a wide array of cardiovascular diseases less invasive catheter-based therapies have become available as an alternative to conventional surgery. Achieving large bore catheter access and vascular access management may be challenging. For large arteriotomies (>12 Fr) suture-based percutaneous closure devices such as Prostar® XL (Abbott Vascular, Santa Clara, CA, USA) or Perclose ProGlide® (Abbott Vascular) are an established technique, yet major vascular complications are relatively common1.
Balloon aortic valvuloplasty (BAV) – with sheath sizes of 10 to 13 Fr – is associated with major vascular complications in 7% of cases2, and with transcatheter aortic valve implantation (TAVI) this rate goes up to 12%3. The ClOsure device iN TRansfemoral aOrtic vaLve implantation (CONTROL) multicentre study, comparing Prostar with ProGlide, showed more major vascular complications with Prostar4. Two thirds of major vascular complications after TAVI result from failed arteriotomy closure5.
Over time, the rate of vascular complications has gradually declined due to growing experience, improved access technique with fluoroscopic or ultrasound guidance and design modifications with smaller profile devices6,7; yet, access-site complications and bleedings are still fairly common and associated with mortality8. Precautionary crossover balloon occlusion may facilitate suture-based closure but requires an additional femoral or radial arterial access and may increase overall procedural costs9.
The percutaneous MANTA Vascular Closure Device (VCD) (Essential Medical Inc., Malvern, PA, USA) is a novel collagen-based technology for closure of large bore arteriotomies. This technical report describes the MANTA design concept, practical instructions for use and preliminary clinical experience.
MANTA design
The MANTA VCD has a closure unit, a delivery system and a dedicated sheath with introducer. There is also a separate 8 Fr puncture location dilator (Figure 1). The puncture location dilator and the MANTA sheath both have a visible metric ruler for assessing the adequate depth. The closure unit comprises the following components: a resorbable polymer (poly-lactic-co-glycolic acid) intra-arterial toggle, an extravascular haemostatic bovine collagen pad, a connecting non-resorbable polyester suture, and a stainless steel suture lock (Figure 2). The delivery system has a tube containing the closure unit and a device handle with a tension gauge to release the closure unit from the tube. The MANTA system comes in a 14 Fr or 18 Fr size. The 14 Fr MANTA VCD is indicated for closing punctures of 10 to 14 Fr, the 18 Fr MANTA VCD for punctures of 15 to 22 Fr.
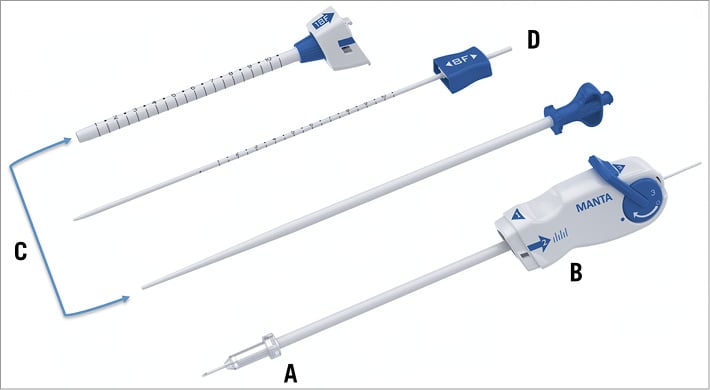
Figure 1. MANTA closure device. Shown are closure unit (A), delivery system (B), dedicated sheath with introducer (C), and 8 Fr puncture location dilator (D). The sheath and location dilator have centimetre markers.
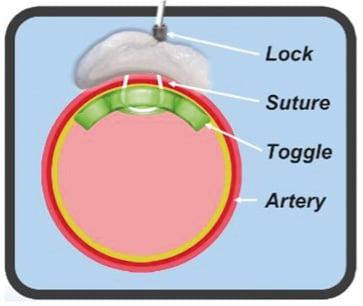
Figure 2. MANTA concept. A bovine collagen pad (in grey) seals the arteriotomy from the outside of the vessel and is connected with the endoluminal bioresorbable toggle through a suture that is closed by a stainless steel suture lock.
Technique
Ultrasound or fluoroscopy guidance may help to achieve a median anterior vessel entry. Angiography should confirm access quality. Pre-closure is not necessary with the MANTA system. Figure 3, Online Figure 1 and Moving image 1 illustrate the procedural steps with MANTA closure. After a 0.035” guidewire and 6 Fr sheath are introduced into the common femoral artery, the sheath is exchanged for the 8 Fr puncture location dilator to measure the length of the subcutaneous track from the skin to the endovascular lumen. Pulsatile blood emerges when the dilator tip is in the arterial vessel. The operator pulls back the dilator until the blood flow ceases and the depth of the vessel lumen can be read from the metric ruler. Empirically 2 cm is added to assure endoluminal deployment of the toggle during the actual MANTA delivery. The arterial access is then up-scaled to the proper sheath size to perform the index procedure. At the end of the index procedure, significant subcutaneous blood collection should be excluded before using the MANTA VCD because significant haematoma could alter the puncture depth relative to the state at baseline. The procedural sheath is exchanged over a 0.035” guidewire for the dedicated MANTA sheath. The sheath introducer is removed and the MANTA closure unit is advanced over the wire into the sheath until the MANTA delivery hub snaps the sheath hub and a clicking sound will be heard. The MANTA sheath closure unit assembly is then slowly withdrawn at a 45° angle with the right hand while providing slight left hand counter push to the skin level to avoid skin tenting. At the predetermined deployment level, the toggle is released by rotating the lever of the MANTA delivery handle in a clockwise direction. The assembly is then slowly and gently withdrawn from the patient. Pulling force can be monitored by a colour code. When excessive force is applied the colour code will switch from green to red, accompanied by an audible “click”. As the MANTA sheath clears the skin layer a blue tamper tube emerges from the deployment tube. Digital left hand pressure around the puncture site is released to advance the tamper tube down along the suture line and secure the stainless steel lock onto the vessel to compact the collagen pad further. The black suture marker becomes visible to indicate full compaction of the collagen. At this point, the arterial wall is sandwiched between toggle and collagen. Tension on the assembly is released and the tamper is slid up the suture line out of the puncture tract. When haemostasis is confirmed, the guidewire is removed. If needed, a final tamp with tension on the handle and monitored pressure on the tamper (green colour at tension gauge) can be performed to ensure complete haemostasis. The suture is cut above the blue tamper and at skin level.
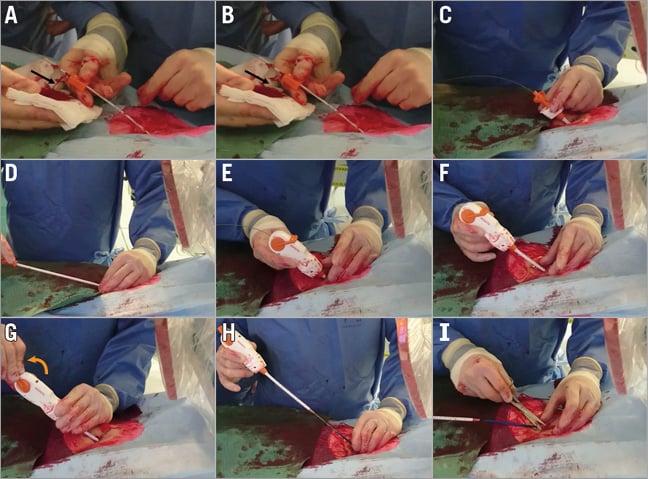
Figure 3. MANTA closure in real practice. A) Puncture depth estimation with the 8 Fr puncture location dilator. Note (pulsatile) blood exiting on the side (black arrow). B) The location dilator is re-advanced and blood ceases (black arrow). C) The MANTA sheath is introduced over a 0.035” guidewire. D) The sheath introducer is removed. E) The MANTA closure unit is advanced over the wire into the sheath. F) The MANTA sheath closure unit assembly is slowly withdrawn at a 45° angle with the right hand while providing slight left hand counter push to the skin level to avoid skin tenting. G) The lever is rotated (orange arrow) to release the toggle. H) The assembly is slowly withdrawn from the patient along the angle of the initial puncture. Pulling force can be monitored by the colour code of the tension gauge and the tamper tube is advanced down along the suture line. I) The suture is cut above the tamper and at skin level.
The MANTA components will resorb in six months. In the event that the patient requires re-access within six months from MANTA closure, X-ray should be used to identify the radiopaque lock, and the common femoral artery should be punctured at least 2.5 cm from the present MANTA device.
MANTA closure should be used with caution at puncture sites: 1) in the proximity (<1 cm) of large bifurcations, 2) with significant atherosclerotic disease or circumferential calcifications, or 3) with non-central vessel entry.
Initial Rotterdam experience
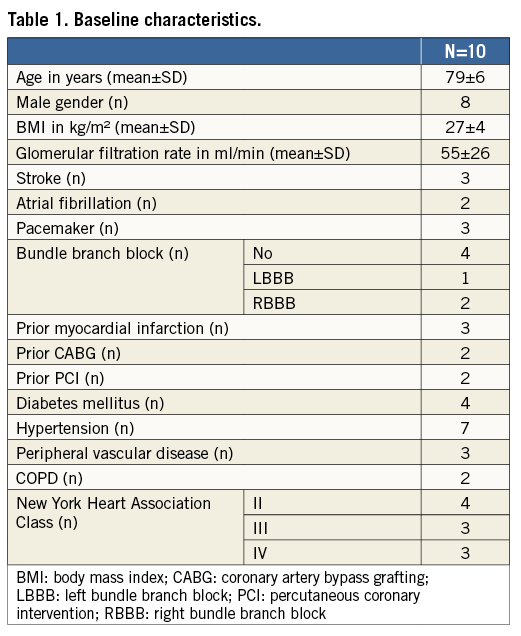
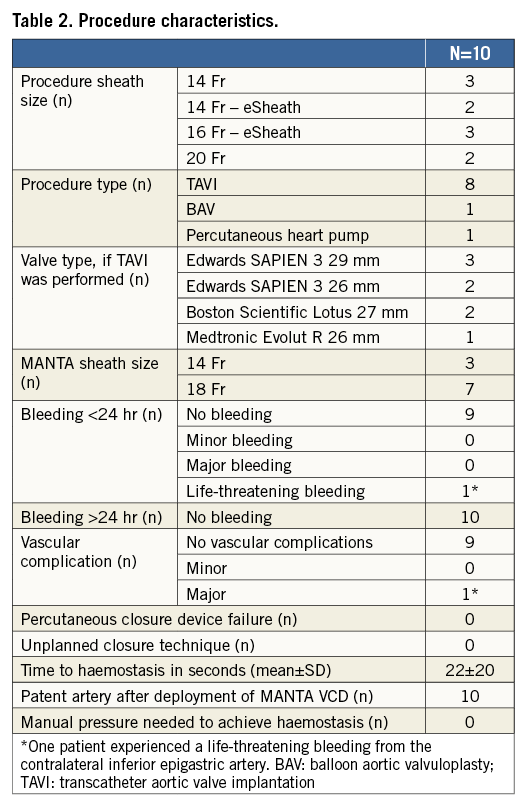
MANTA closure was applied in 10 consecutive patients undergoing various large bore catheter interventions, including TAVI (n=8), BAV (n=1) and high-risk percutaneous intervention with a 14 Fr circulatory support device (n=1). All patients underwent pre-procedural planning by MSCT and/or ultrasound plus ad hoc angiography to assess the iliofemoral arterial tree in terms of size, atherosclerotic disease, calcium distribution and tortuosity. Baseline patient characteristics are displayed in Table 1. Mean age was 79±6 years, and eight of the 10 patients were male. Procedural characteristics are shown in Table 2. Sheath size closed with the MANTA device varied from outer diameter 14 Fr to 22 Fr, including the self-expanding eSheath technology (Edwards Lifesciences, Irvine, CA, USA) in five patients. The 14 Fr and 18 Fr MANTA were used in three and seven patients respectively. MANTA access closure was successful in all patients. Time from toggle release (i.e., start of device deployment) to haemostasis was 22±20 seconds. Angiography confirmed a patent artery in all cases. There were no relevant bleeding or vascular complications after MANTA closure. One patient experienced a major vascular complication due to laceration of the inferior epigastric artery in the contralateral access site that was not related to the MANTA VCD.
Conclusion
Early clinical experience is promising but needs confirmation in larger clinical trials. Results from the CE-mark study (NCT 02521948) to evaluate the safety and performance of MANTA VCD are awaited in 2016.
| Impact on daily practice The MANTA VCD is a novel collagen-based closure device, simple to use and applicable in a variety of cardiovascular interventions requiring large bore catheters between 10 and 22 Fr. |
Conflict of interest statement
N.M. Van Mieghem has received research grants from Claret Medical, Boston Scientific, Medtronic and Edwards Lifesciences. P.P.T. de Jaegere is a proctor for Boston Scientific. G. Roubin is Chief Medical Officer of Essential Medical Inc., with equity interest. G. Walters is Chief Executive Officer of Essential Medical Inc. T. Sorzano, T. Grintz and S. Nardone are engineers at Essential Medical Inc. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Typical MANTA closure.
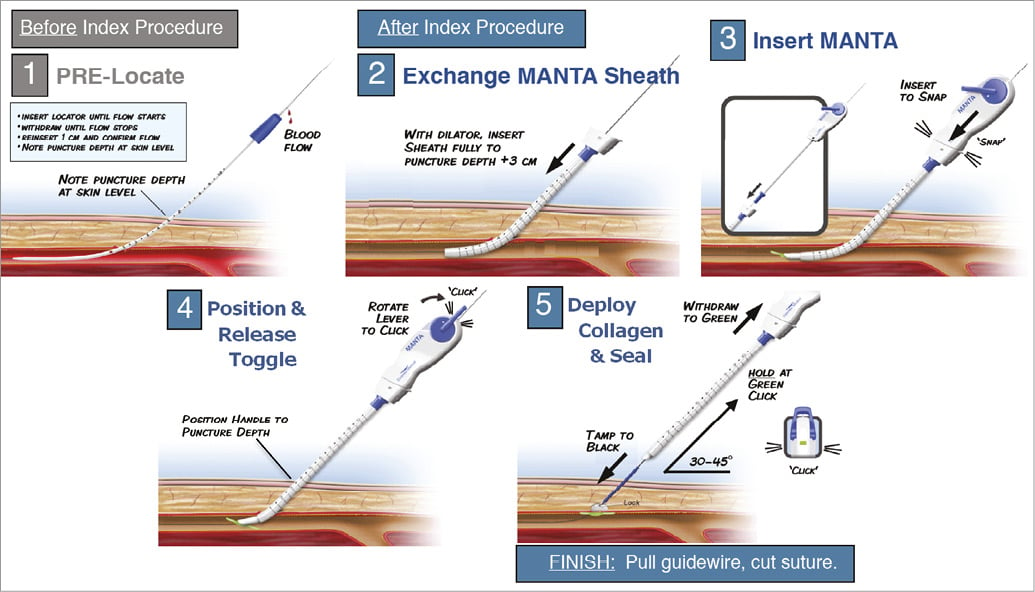
Online Figure 1. Typical MANTA closure.
Supplementary data
To read the full content of this article, please download the PDF.
Typical MANTA closure.

