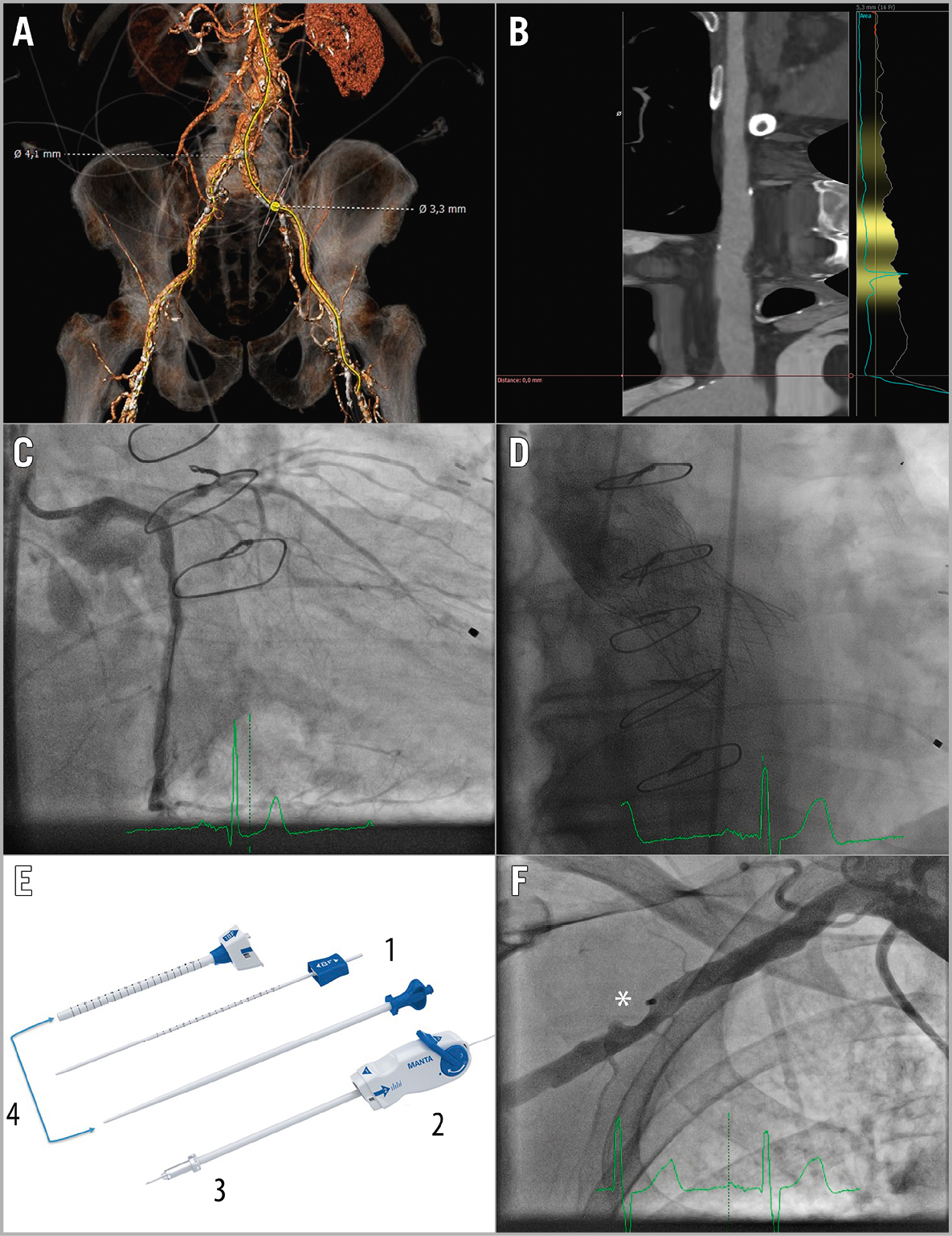
Figure 1. Planning and performing plug-based closure in percutaneous right-sided transaxillary TAVI. A) No safe femoral access due to small diameters. B) Right subclavian artery. C) Result after successful LM stenting. D) Post-deployment angiography of the CoreValve Evolut R XL. E) Puncture location dilator (1); delivery system (2); closing unit (3); dedicated MANTA sheath and introducer (4). F) Final angiography after MANTA closure. *Endoluminal bioresorbable toggle
An 82-year-old male patient was referred to our hospital because of dyspnoea on minimal exertion. Diagnostic investigations showed a severe aortic valve and distal left main (LM) stenosis. A multislice computed tomography scan demonstrated no safe femoral access (diameters of 3.3 mm and 4.1 mm) (Figure 1A). The Heart Team reached consensus for transcatheter aortic valve implantation (TAVI) combined with percutaneous coronary intervention (PCI). Access through the right subclavian artery was preferred (Figure 1B), because of the presence of a LIMA graft.
Completely percutaneous transaxillary TAVI is feasible1. Transaxillary access was utilised under continuous ultrasound guidance (Moving image 1). Angiography images show the result after successful LM stenting and CoreValve® Evolut™ R XL valve (Medtronic, Minneapolis, MN, USA) deployment (Figure 1C, Figure 1D). Currently, percutaneous closure of large bore arteriotomies can be achieved through suture and plug-based closure. However, suture-based closure comes with a major vascular complication rate of approximately 14% with two thirds of them caused by closure device failure2.
We performed arteriotomy closure with the MANTA™ device (Essential Medical, Inc., Exton, PA, USA). It consists of an endoluminal bioresorbable toggle and a collagen plug outside the vessel connected together with a suture and a stainless steel lock. Step-by-step deployment has been described previously3. In short, before the index procedure the arteriotomy depth must be measured with the puncture location dilator (Figure 1E1, Moving image 2). After performing the index procedure, the delivery system with the closing unit (Figure 1E2, Figure 1E3) is clicked on the dedicated MANTA sheath (Figure 1E4). We inserted a femoral safety wire in case it was needed for bail-out situations (i.e., balloon dilatation or a covered stent) (Moving image 3). The delivery system is then pulled back up to the arteriotomy depth measured previously, and the toggle can then be released. A blue tamper tube rises up which can be advanced over the wire to tighten up the collagen plug to the toggle (Moving image 4). In our case immediate haemostasis was reached. Note the presence of the endoluminal bioresorbable toggle (Figure 1F) which will resorb completely within six months.
In conclusion, plug-based closure with the MANTA device may be feasible in completely percutaneous right-sided transaxillary TAVI.
Funding
The Erasmus Medical Center has received research grants from Essential Medical.
Conflict of interest statement
N. Van Mieghem is a member of the advisory board of Essential Medical. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Ultrasound-guided percutaneous transaxillary access.
Moving image 2. Arteriotomy depth measurement.
Moving image 3. Inserting the femoral safety wire.
Moving image 4. Successful transaxillary closure with the MANTA device.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Ultrasound-guided percutaneous transaxillary access.
Moving image 2. Arteriotomy depth measurement.
Moving image 3. Inserting the femoral safety wire.
Moving image 4. Successful transaxillary closure with the MANTA device.

