Abstract
Transcatheter aortic valve implantation (TAVI) is a widely accepted alternative to surgical aortic valve replacement (SAVR) among non-operable patients or selected high-risk patients with degenerative, severe aortic stenosis. TAVI is considered less invasive when compared with SAVR; however, there remain significant differences between different TAVI access routes. The transfemoral approach is considered the least invasive access route, and can be performed as a fully percutaneous procedure in a spontaneously breathing patient under local anaesthesia and mild sedation only. Moreover, transfemoral TAVI patients are typically transferred to coronary care rather than to an intensive care unit after the procedure, and benefit from early ambulation and a reduction in overall length of hospital stay. Considering these patient-specific and health-economic advantages, several TAVI centres follow the least invasive strategy for their patients and have implemented the transfemoral access route as the default access in their institutions. This article provides an overview on the prerequisites for a successful transfemoral TAVI procedure, describes the procedural advantages compared to alternative access routes, and highlights differences in clinical outcomes.
Introduction
Degenerative aortic valve stenosis is clinically the most relevant valvular heart disease in the ageing patient population1. The prevalence of severe aortic stenosis is as high as 3.4% among elderly patients, and up to 76% are considered symptomatic2. Surgical aortic valve replacement (SAVR) has been the established standard in the treatment of symptomatic patients for decades, as it effectively relieves symptoms, restores health-related quality of life and improves prognosis3. Patients undergoing SAVR benefit from the experience of more than 50 years of development and refinement of surgical techniques and valve designs and, by virtue of these advantages, they face low rates of perioperative complications and favourable clinical outcomes. Despite these advantages, conventional SAVR still requires sternotomy, cardioplegic arrest and the use of the heart-lung machine, and this constitutes a major surgical intervention taking the corresponding time for recovery. Since particularly elderly patients face comorbidities leading to an increased risk for SAVR, a substantial number of patients are not referred for surgical therapy and are left untreated4.
TAVI evolution and current state of the art
The early experience of transcatheter aortic valve interventions was achieved by percutaneous transvenous valve delivery. However, this access turned out to be technically challenging and associated with a variety of complications. As a consequence, the concept of a transarterial, transfemoral retrograde access was developed, optimised and simplified over time to become the standard transfemoral access route. Retrograde implantation of transcatheter bioprostheses following successful femoral artery puncture was first described by Webb et al using a balloon-expandable prosthesis5, and by Grube et al for a self-expandable TAVI bioprosthesis6. In parallel to this, an antegrade valve delivery approach using an anterolateral thoracotomy and transapical insertion of the TAVI system was proposed as an alternative access route, particularly suited for patients considered inappropriate candidates for transfemoral TAVI7. In highly selected patients with small peripheral artery diameters or heavily diseased peripheral vasculature in combination with anatomical specifications precluding a transapical insertion of a TAVI delivery system, direct exposure of the subclavian artery6,8, the ascending aorta9,10, or the carotid artery are used to introduce transcatheter heart valve systems and treat degenerative aortic valve stenosis11 (Table 1).
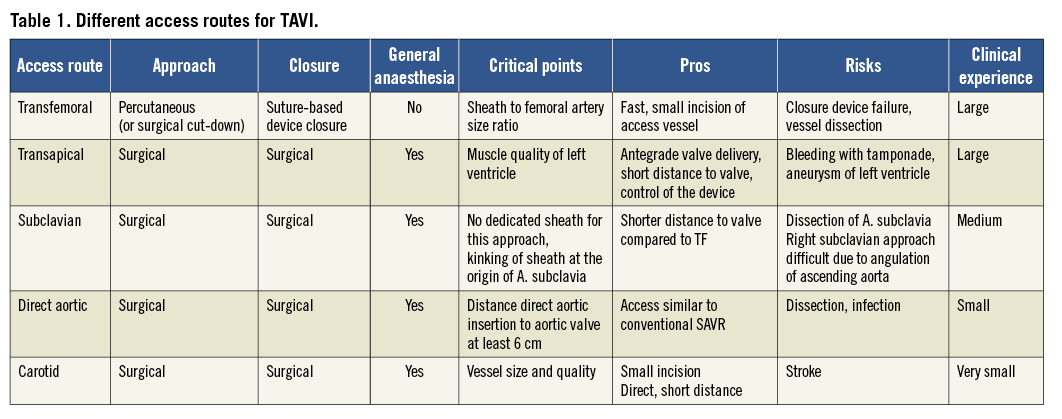
In contemporary clinical practice, the transfemoral access route is the preferred access in numerous TAVI centres and is applied in the majority of the procedures (>70%), whereas the transapical (approximately 20%), the subclavian and direct aortic access (approximately 10%) are considered alternative access routes12.
Anatomical prerequisites and procedural considerations for transfemoral TAVI
Preprocedural imaging using different modalities is required in order to thoroughly evaluate the individual anatomical characteristics and identify the most appropriate device and access route for the patient. Apart from information on aortic annulus size, calcification and geometry, detailed information on the peripheral vasculature is acquired. During preprocedural coronary angiography, additional invasive access route evaluation by contrast injection into the femoro-iliac vasculature and the aortic root provides some first insights into the feasibility of transfemoral TAVI. In addition, contrast enhanced multislice computed tomography imaging with three-dimensional reconstruction is warranted and provides a detailed understanding of peripheral vessel diameter, the grade and distribution of vascular calcification as well as vascular tortuosity (Figure 1)13.
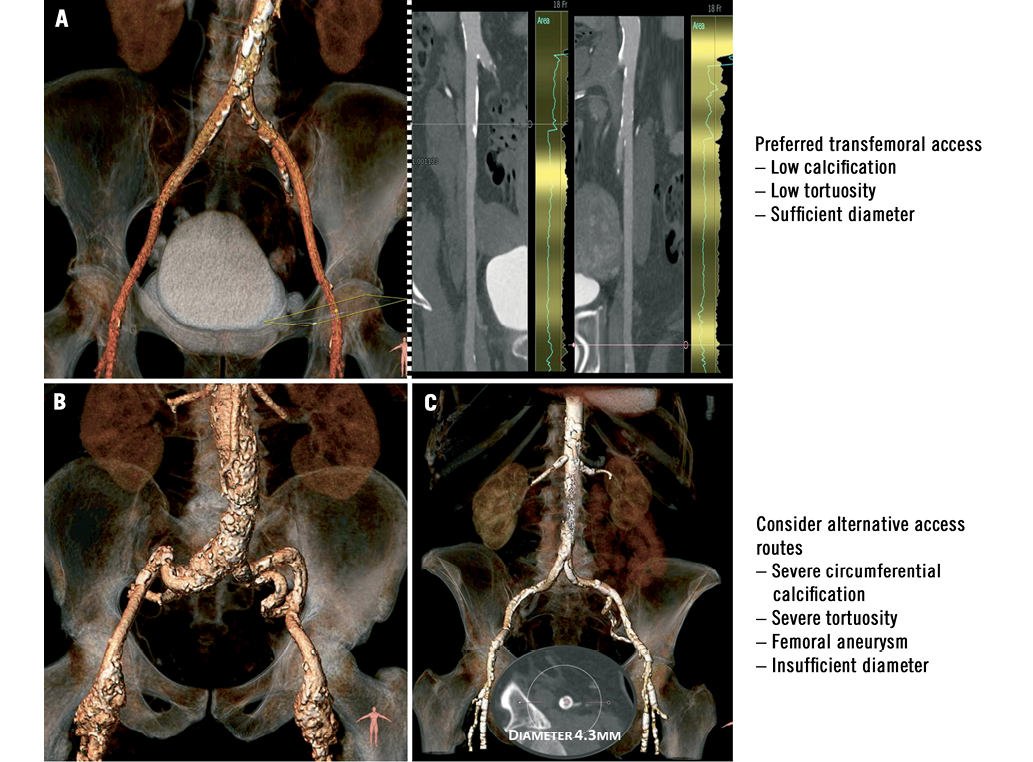
Figure 1. Preprocedural access site screening with multislice computed tomography and three-dimensional reconstruction. A) Ideal peripheral vasculature for transfemoral TAVI; B) severe tortuosity of iliac vessels and femoral aneurysms – alternative access should be considered for TAVI; C) severe, circumferential calcification of femoral arteries and insufficient vascular dimensions to accommodate a TAVI sheath – alternative access should be considered for TAVI.
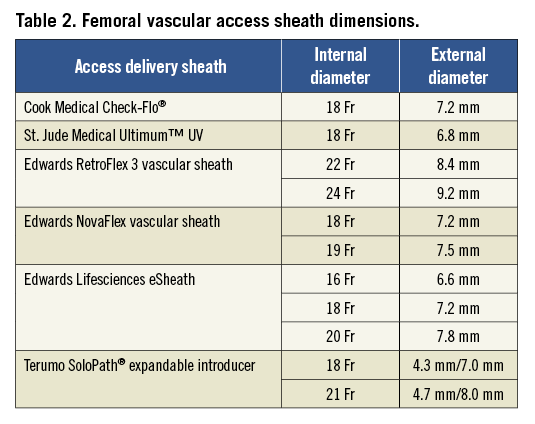
The selection criteria for early-generation TAVI devices were mainly dependent on the size of the femoral vasculature, as dimensions of at least 8.4 mm and 9.2 mm were required to safely accommodate a 22 Fr and 24 Fr delivery sheath. In order to avoid femoral vascular injury, an outer sheath to femoral artery minimal lumen diameter ratio (SFAR) greater than or equal to 1.0514 should be considered in the preprocedural planning phase. While this ratio can be extended to 1.10 in the absence of severe femoral calcification, it decreases to 1.00 in the presence of circumferential calcium. Newer-generation TAVI devices have overcome this limitation and in contemporary clinical practice the majority of transcatheter aortic valve devices are delivered through 16 Fr to 19 Fr vascular sheaths which require femoral vascular diameters of 6.6 mm to 7.5 mm, respectively. The latest developments in transcatheter aortic valve interventions have focused on the reduction of delivery catheter profile, as in the Edwards SAPIEN 3 transcatheter heart valve system profile which was reduced to 14 Fr (eSheath; Edwards Lifesciences, Irvine, CA, USA), despite the integration of a dedicated sealing skirt to reduce paravalvular regurgitation. By using the concept of dynamic, transient sheath expansion during the passage of the TAVI delivery catheter, the eSheath aims to reduce vascular wall stress, thereby minimising vascular injury and access-related vascular complications. Specific sheath types have been developed in order to facilitate femoral treatment in case of a narrow iliofemoral vasculature, like the SoloPath® sheath (Terumo Corp., Tokyo, Japan) which is inserted as a 13-14 Fr sheath and expanded by inflation of a balloon up to 21 Fr. The most commonly used vascular access sheath types with their respective sizes are displayed in Table 2.
Transfemoral access
Owing to the large delivery sheath diameters of early-generation TAVI devices, surgical cut-down was frequently performed to enter the femoral vasculature safely under direct visualisation and ensure uncomplicated surgical vascular closure after successful device implantation. However, newer-generation and lower-profile TAVI devices can be delivered safely using a complete percutaneous procedure15. The selection of the puncture site is crucial in order to minimise vascular injury and should be located above the femoral bifurcation in a vascular segment with little or no calcification. The identification of this area can be performed either with vascular ultrasound, contralateral pigtail injection, additional ipsilateral puncture and dye injection or by contralateral pigtail catheter delivery, which can be used as marker for appropriate vessel puncture. After successful vascular puncture and retrograde insertion of a standard 0.035 inch wire, the femoral artery is predilated with a 9-10 Fr vascular introducer sheath in order to accommodate the Perclose suture device (Abbott Vascular, Santa Clara, CA, USA).
The exchange of the standard wire to a stiff wire provides sufficient support during sheath insertion, which is done under fluoroscopic guidance. Following successful TAVI implantation and delivery catheter removal, vascular access site closure might be facilitated by a vascular crossover technique prior to closure with the suture device16. After advancing a flexible 6-7 Fr crossover sheath from the contralateral access site, a peripheral balloon is inflated proximal to the delivery sheath to block the flow in the iliac artery temporarily. Either one ProStar XL® (Abbott Vascular)17, or two ProGlide® (Abbott Vascular) devices are used to achieve sufficient haemostasis after TAVI delivery sheath removal18.
Residual bleeding, dissection or perforation of the iliofemoral vasculature can easily be assessed with an injection through the contralateral sheath. If needed, a balloon dilatation to seal a dissection or insertion of a covered stent can easily be performed with this setup. Alternatively, an additional ipsilateral puncture of the femoral artery might help to check the result of vascular access site closure.
Advantages of transfemoral TAVI
Compared to SAVR, the advantages of transcatheter treatment of severe aortic stenosis are mainly explained by the less invasive nature of the procedure. Transfemoral TAVI is considered the least invasive approach to treat degenerative aortic stenosis effectively. To keep the procedure as minimally invasive as possible, TAVI might be performed as a purely percutaneous procedure in a conscious patient under local anaesthesia19. This results in a reduction of procedure time and overall hospital length of stay, as patients do not require intensive care unit treatment and are mobilised the day after intervention20. Furthermore, a percutaneous, transfemoral access might be associated with a reduction in post-procedural pain21, and less delayed wound healing or wound infection in comparison with alternative access routes22. All these advantages have a substantial impact on health-economic outcomes. Evaluating the direct incremental cost-effectiveness of TAVI compared to medical therapy in the PARTNER B trial, transcatheter valve treatment was associated with higher medical care costs during the initial hospitalisation, but lower costs during the first year because of reduced repeat hospitalisations. Cumulative costs were almost doubled compared to medical therapy during the first year after the procedure; however, the incremental cost-effectiveness ratio for TAVI per quality-adjusted life year gained was located well within the established ranges of willingness-to-pay23. Comparing the direct cost-effectiveness of TAVI with SAVR in the PARTNER A trial, similar one-year costs and quality-adjusted life years (QALYs) were observed24. However, a stratified subanalysis according to access route pointed to a significant difference of health-related costs during the first year after TAVI when comparing transfemoral and transapical implantation of TAVI. TAVI using the transapical access resulted in higher costs and less quality-adjusted life expectancy compared with SAVR. However, transfemoral TAVI appeared to be attractive from an economical point of view with lower costs for one year after TAVI and higher health-adjusted life expectancy when compared to transapical TAVI and SAVR.
Clinical outcomes
TAVI, although less invasive than SAVR, might be associated with significant and potentially life-threatening complications. The evaluation of these complications and the assessment of severity should be performed in a standardised fashion. In the absence of uniform endpoint definitions, in 2011 the Valve Academic Research Consortium (VARC) created a consensus document to standardise TAVI-specific outcomes and provide comparable endpoint definitions25. After one year of experience in the utilisation of these clinical endpoints, certain definitions were revisited and updated in 2012 according to contemporary clinical practice (VARC 2)26.
At this point in time, the available evidence on TAVI treatment is based on prospective cohort studies reflecting the experience of selected TAVI centres and on randomised controlled trials comparing either TAVI with standard medical therapy, or TAVI with SAVR. Although there is controversy and an ongoing debate on the apparent impact of access route selection itself, evidence from a direct comparison of outcomes after transfemoral TAVI or TAVI with alternative access routes is still lacking.
Per protocol, patients in PARTNER B were only treated using the transfemoral access route, whereas in PARTNER A, transapical placement was allowed in cases where peripheral artery anatomy precluded a femoral valve delivery. Keeping in mind that randomisation in the PARTNER trial was not stratified by access and more than 70% of TAVI patients underwent a transfemoral procedure, substantial differences between access routes have been observed for all-cause mortality at 30-day (TF vs. TA: 3.7% vs. 8.7%) and at one-year follow-up (21.3% vs. 29.1%) in the “as treated” analysis of PARTNER A. While the difference in outcome was mainly explained by the differences in baseline risk profile, the debate on the safety profile of alternative access routes and their impact on outcomes has started.
After the overall promising results from the PARTNER trial, the worldwide number of TAVI procedures has increased and the range of patients being offered this procedure has been extended to particular lower-risk patients27,28. In anticipation of similar outcomes between TAVI and SAVR even in the low-risk, operable patient population, the STACCATO trial was initiated comparing transapical TAVI with SAVR29. A total of 200 patients were planned to be randomly allocated to either transapical TAVI or SAVR; however, an excess of significant and potentially life-threatening complications in the transapical TAVI arm led to premature study termination following advice from the Data Safety Monitoring Board.
Several cohort studies and large post-market registries investigating high-risk TAVI patients in a real-world setting tried to stratify outcomes according to access routes; however, the significance of the findings was limited by differences in baseline clinical characteristics and clinical risk profiles30,31. Differences in survival at one year after TAVI favouring patients undergoing transfemoral TAVI in the SOURCE registry (transfemoral vs. transapical; 81.1% vs. 72.1%) were also explained by significant differences in baseline characteristics. However, after adjustment for these confounders in the SOURCE XT registry, transfemoral TAVI patients had better clinical outcomes up to one year after TAVI and the transapical access has been identified as an independent predictor for mortality (transfemoral vs. transapical: 85.0% vs. 72.8%, HR 1.64, 95% CI: 1.28-2.09, p<0.0001)32.
The most frequent complication after transfemoral TAVI is vascular access site complications. While vascular injury is correlated with the size of the delivery sheath diameter, the rate of access site complications could be reduced from more than 30% with early-generation TAVI devices33,34 to 9% in contemporary clinical practice15. Major vascular complications are associated with major bleeding, transfusion of red packed blood cells, and renal failure requiring dialysis, and have an impact on clinical outcomes35. Procedural modifications, improvement of suture-based vascular closure devices as well as continuous refinement of delivery catheter design and profile will help to reduce vascular complications further. In case of vascular injury or incomplete vascular access site closure, the implantation of a covered stent graft is associated with high rates of immediate closure success and patency during follow-up36.
The most unbearable events from a patient’s perspective are cerebrovascular events, as they might be associated with significant morbidity and persistent disability. Observational studies suggested a higher risk of cerebral embolism during transfemoral TAVI37, which might be explained by the manipulation of the bore, delivery catheter in the aortic arch and the ascending aorta. However, neither an increase in silent cerebral ischaemic lesions38 nor of clinically apparent stroke events was observed when comparing transfemoral and transapical patient cohorts39,40. Of particular note, the risk of new-onset atrial fibrillation appears to be elevated in transapical compared with transfemoral procedures which might translate into a higher rate of stroke in the longer-term observational period (transapical OR 4.08, 95% CI: 1.35-12.31, p=0.019)41.
Irrespective of access route, TAVI improves health-related quality of life during the first year after TAVI42,43. Among inoperable patients undergoing transfemoral transcatheter valve treatment, TAVI provided substantial benefit over medical therapy in terms of symptoms and health-related quality of life44. While health-related quality of life more rapidly improved after transfemoral TAVI as compared with SAVR, no difference was observed when transapical TAVI was performed. After six months there was no significant difference between TAVI, irrespective of access, and SAVR with respect to health status and health-related quality of life45.
Future outlook
In contemporary clinical practice the majority of TAVI centres follow the strategy of the least invasive approach and consider the transfemoral access route as their default access. Only patients with unsuitable peripheral vasculature diameters or significant peripheral artery disease with relevant stenoses, calcification, tortuosity and aneurysms should be considered for an alternative access such as the transapical, subclavian, direct aortic or carotid approach. While nowadays more than 70% of selected high-risk patients are considered appropriate candidates for transfemoral TAVI, forthcoming technological advances and innovations in transcatheter valve technology may facilitate further transfemoral delivery of TAVI devices and increase the number of transfemoral-treated patients.
While the treatment of intermediate-risk patients is not recommended at this point in time, the reduction in transfemoral delivery catheter profile and heart valve design will help to extend further the indication for this therapeutic option. Currently, two randomised controlled trials are investigating the safety and efficacy of TAVI using either the Medtronic CoreValve (SURTAVI trial) or the Edwards SAPIEN XT bioprosthesis (PARTNER II) compared to SAVR in intermediate-risk patients.
Conclusion
Transcatheter aortic valve interventions are preferably performed using the transfemoral access route and local anaesthesia only. In experienced hands, a transfemoral TAVI procedure requires less than one hour of procedure time, avoids intensive care unit treatment and allows for early mobilisation, thereby reducing hospital length of stay and healthcare costs. However, vascular complications after transfemoral TAVI remain a matter of concern due to their impact on clinical outcomes. Technical improvement and refinements in prosthesis design and delivery catheter profile will help to minimise this risk. Alternative access routes will remain as additional treatment options for patients with unsuitable iliofemoral vasculature.
Funding
Supported by an unrestricted research grant from Medtronic to the University of Bern (PW, COS).
Conflict of interest statement
P. Wenaweser is proctor and receives honoraria from Medtronic and Edwards Lifesciences. S.Windecker has received honoraria and consultant fees from Edwards Lifesciences and Medtronic. L. Buellesfeld is a consultant and proctor for Medtronic and Edwards Lifesciences. The other authors have no conflicts of interest to declare.

