
Abstract
Aims: A transfemoral transarterial approach is considered the preferable access route for transcatheter aortic valve implantation (TAVI), followed by a transaxillary/subclavian TAVI approach. However, these approaches may not be an option in all patients. This study aimed to report the initial European experience with transfemoral transcaval TAVI.
Methods and results: Data on 50 patients treated by transcaval TAVI in five European centres were collected and analysed according to the Valve Academic Research Consortium (VARC)-2 definitions. The study population had a mean age of 78.7±8.0 years and a high surgical risk profile (median STS risk score 6.1%, interquartile range 3.0-11.2%). Transcaval access was successful in 49 out of 50 patients and device success was obtained in 94% of cases. Closure of the caval-aortic puncture site with a nitinol cardiac occluder was successful in all cases without need for emergent surgery. One patient received additional sealing of the aortic puncture site with a covered stent one day post TAVI due to a gradual haemoglobin drop of 3 g/dL. VARC-2-defined life-threatening bleeding and major vascular complications possibly related to transcaval access were 4% and 10%, respectively. There were no bleeding or vascular complications after discharge. At 30 days, the clinical efficacy endpoint was reached in 88% of patients.
Conclusions: Transfemoral transcaval access proved to be a feasible and safe TAVI approach for high-risk patients with severe aortic stenosis not suitable for transfemoral or transaxillary/subclavian transarterial access.
Introduction
Transcatheter aortic valve implantation (TAVI) has evolved into a safe and effective procedure with predictable and reproducible outcomes. Whilst a transfemoral (TF) transarterial approach is considered the preferable access route, this approach may not be an option in all cases. Owing to inferior outcomes with transapical and direct aortic approaches, most operators try to avoid a transthoracic approach. Alternative transarterial access strategies including transaxillary/subclavian and transcarotid access have increasingly been used for TAVI over the past few years.
Since its first description in 2014 by Greenbaum and colleagues1, the transcaval approach has attracted increasing attention as a further percutaneous, transvascular TAVI option for patients with challenging access. This study reports on the feasibility and safety of this alternative TAVI access in the initial experience of five experienced European TAVI centres.
Methods
PATIENT SELECTION AND DATA COLLECTION
Between December 2014 and May 2019, fifty patients with severe native aortic valve stenosis or a degenerated surgical aortic bioprosthesis underwent TAVI by transcaval access in five European TAVI centres (Supplementary Table 1),2. Preprocedural multislice computed tomography (MSCT) scans were available for all cases and were evaluated locally, confirming both the unsuitability for transfemoral/axillary/subclavian access and the fulfilment of anatomical criteria for a transcaval access3. All patients were discussed at the local Heart Team meeting and transcaval TAVI was considered the preferred option. The MSCT scans were used to determine the most favourable calcium-free caval-aortic puncture site in the infra-renal aorta (Figure 1). The choice of valve type and size was at the discretion of the operator. Transcaval TAVI procedures were performed as previously described4. A description of tools and techniques used for transcaval TAVI is provided in Figure 2. Adverse events and procedural and clinical endpoints were assessed according to the Valve Academic Research Consortium (VARC)-2 definitions5. Baseline and post-procedural echocardiographic data were collected for all patients. A clinical follow-up was obtained at 30 days. The study was conducted in accordance with the Declaration of Helsinki and all patients gave written informed consent prior to anonymous data collection.
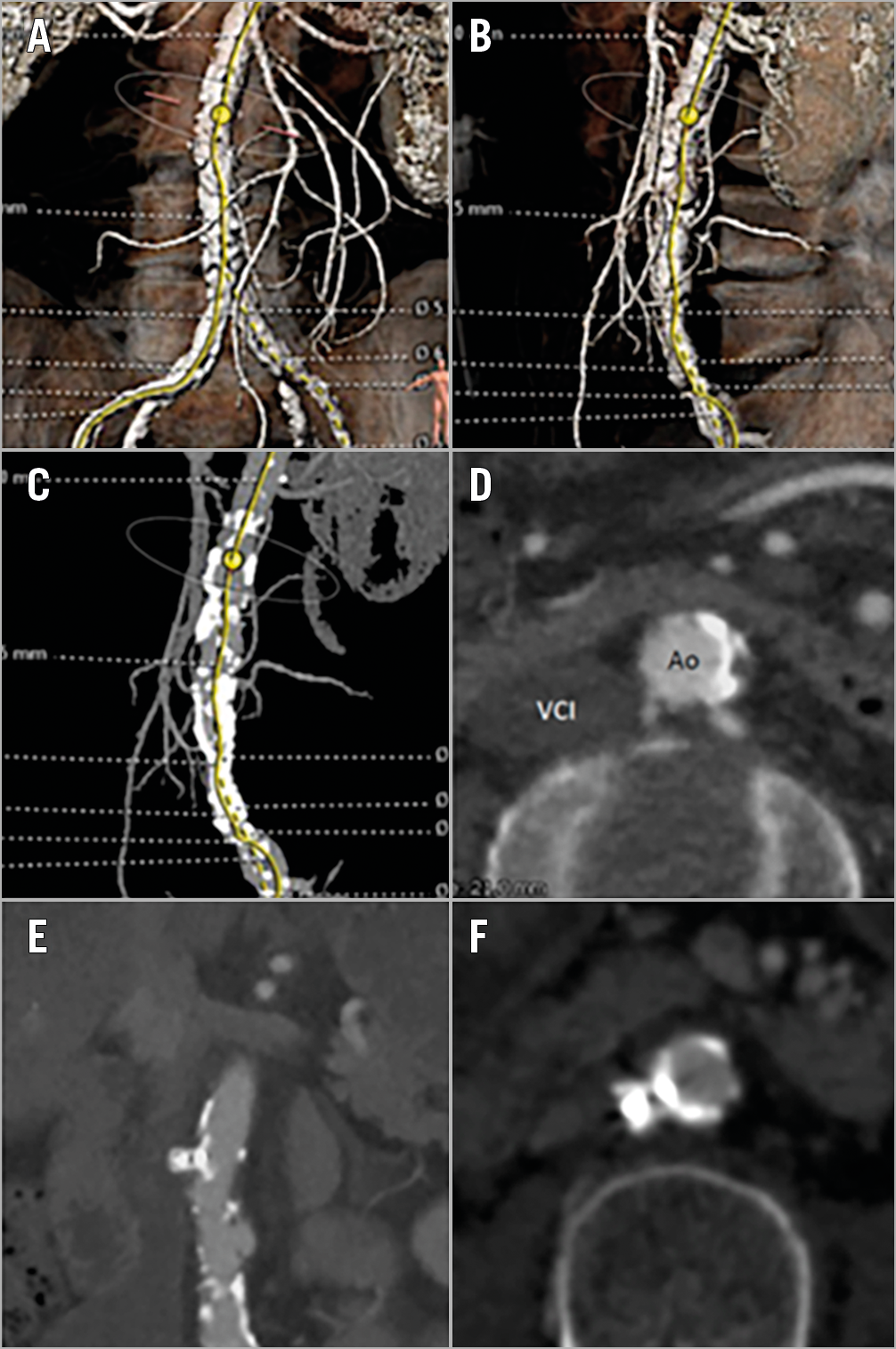
Figure 1. MSCT imaging. A) - D) Three- and two-dimensional MSCT imaging of the abdominal aorta showing a calcium-free segment without interposed structures, suitable as a target for caval-aortic puncture. E) & F) Complete closure of the caval-aortic puncture site with an Amplatzer Duct Occluder as assessed by MSCT one month after transcaval TAVI. Ao: aorta; VCI: inferior vena cava
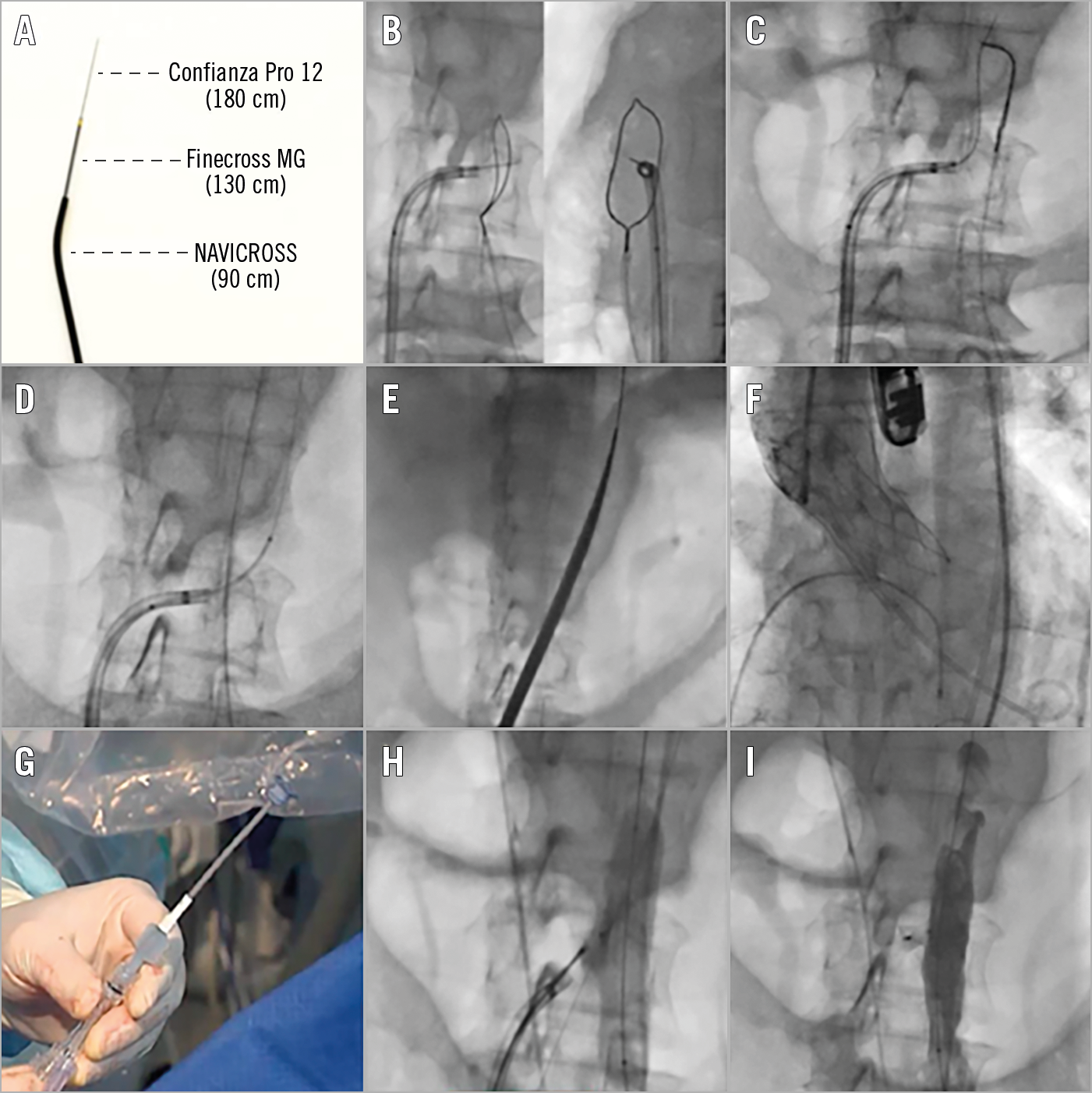
Figure 2. Tools and techniques for transcaval TAVI. A) A crossing system consisting of a Confianza Pro 12 coronary guidewire (Asahi Intecc, Aichi, Japan) with an amputated tip, a Finecross® MG microcatheter (Terumo), and a NAVICROSS® microcatheter (Terumo) can be used. B) A steerable introducer (e.g., 71 cm Agilis™ NxT 8.5 Fr; Abbott Vascular) introduced by the right femoral vein can be used to guide the caval-aortic crossing. A 25 mm gooseneck snare introduced via the femoral artery is placed in the aorta for snaring. An RAO 5-20° projection is typically used to determine the height of the puncture; an orthogonal view gives the operator a “bulls-eye” target. An electro-surgery pencil, clamped to the distal end of the Confianza guidewire, is used for crossing the Confianza guidewire. C) Successful snaring of the Confianza guidewire and pushing the guidewire higher into the aorta descendens. D) Next, the Finecross and NAVICROSS microcatheters are crossed over into the aorta, the latter being compatible with a 0.035” stiff guidewire. E) Introduction of the TAVI introducer sheath. F) Successful TAVI procedure. G) The caval-aortic puncture site is typically closed using a 10×8 mm Amplatzer Duct Occluder; notice the amputated loader of the Duct Occluder. H) The Duct Occluder is delivered through the steerable introducer to ensure coaxial closure. A 14 to 20 mm compliant balloon can be introduced by the femoral artery and inflated at the caval-aortic puncture site to ensure good wall apposition of the Duct Occluder and promote thrombosis inside the occluder. I) An abdominal aortogram is obtained to assess aortic wall closure and/or potential presence of a remaining shunt to the inferior vena cava or retroperitoneal space.
STATISTICAL ANALYSIS
Continuous variables are expressed as mean±standard deviation or as median with interquartile range. Categorical variables are presented in numbers and percentages (%). SPSS Statistical Software, Version 24 (IBM Corp., Armonk, NY, USA) was used for statistical analysis.
Results
BASELINE DATA
In total, 50 patients were included in the analysis. The mean age of the study population was 78.7±8.0 years and 58% were female. The mean Society of Thoracic Surgeons (STS) risk score was 8.6±6.7% (median 6.1%, interquartile range 3.0% to 11.2%). Baseline clinical and echocardiographic data are shown in Table 1.
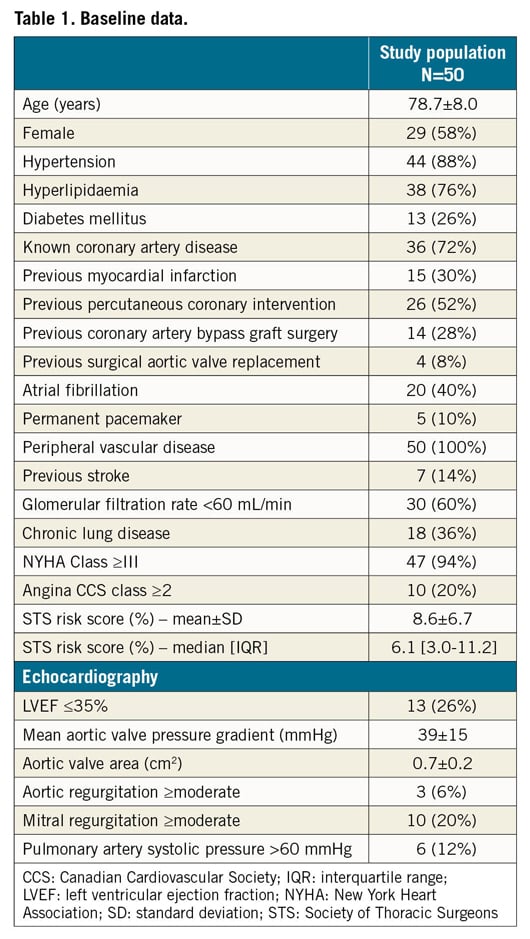
PROCEDURAL DATA AND IN-HOSPITAL OUTCOMES
Most cases (86%) were performed under general anaesthesia and the others under conscious sedation. Half of the patients (52%) were treated with a balloon-expandable transcatheter aortic valve (SAPIEN 3 [Edwards Lifesciences, Irvine, CA, USA], n=26), and the other half with a self-expanding transcatheter aortic valve (Portico™ [St. Jude Medical (now Abbott Vascular), St. Paul, MN, USA], n=13; Evolut™ R/PRO [Medtronic, Minneapolis, MN, USA], n=6; ACURATE neo™ [Boston Scientific, Marlborough, MA, USA], n=5). Mean fluoroscopy time was 34±19 minutes and the mean amount of contrast medium used was 145±58 ml per case. Procedural data are summarised in Supplementary Table 2.
Device success was achieved in 94% of cases. One patient experienced aortic dissection/rupture during sheath passage (which was initially undetected) and died of refractory haemorrhagic shock and cardiac arrest after TAVI completion. There were no valve-related complications (valve embolisation, annular rupture, cardiac tamponade, coronary obstruction or need for conversion to cardiac surgery). Overall valve performance was satisfactory with a transvalvular mean gradient <20 mmHg in all cases and only one case with moderate paravalvular regurgitation (Table 2).
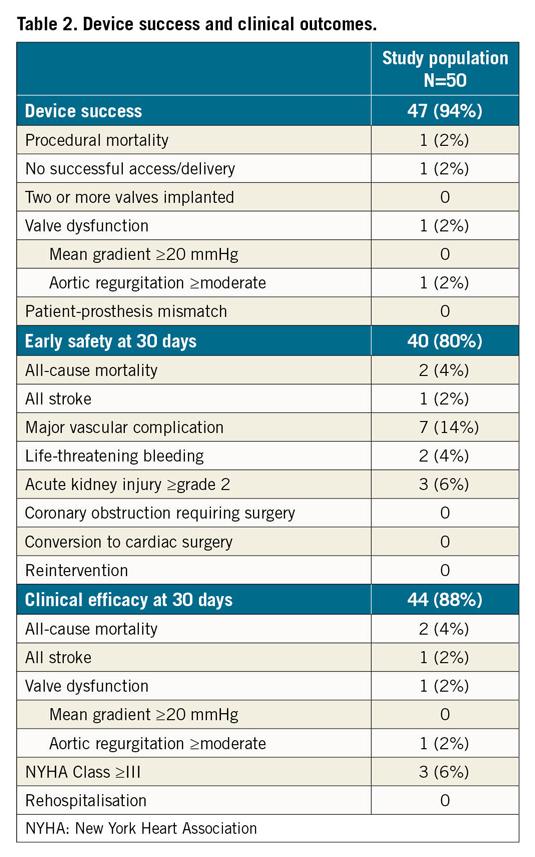
Immediate aortic puncture site closure was judged to be successful in all patients – except for the patient with aortic dissection/rupture – with complete closure in 23 patients (46%) and a funnel-shaped (n=22, 44%) or cruciform-shaped (n=4, 8%) minor leak into the inferior vena cava in the remaining patients. The most frequently used closure device was the Amplatzer™ Duct Occluder (Abbott Vascular, Santa Clara, CA, USA) in 46 patients; in three other patients an Amplatzer™ VSD Occluder (Abbott Vascular) was used. In one patient, the regular closure device could not be used as the SAPIEN implantation balloon had ruptured and could not be retrieved into the delivery sheath, forcing removal of the entire system including the introducer sheath. This case required implantation of a covered stent (Ovation® iX; Endologix, Inc., Irvine, CA, USA) over the puncture site, which was successful and avoided a major vascular complication (Supplementary Table 2).
Overall, seven VARC-2-defined major vascular complications were noted. Two cases of major vascular complication were associated with a life-threatening bleeding: the patient with an aortic dissection/rupture during sheath passage (as described above), and one other patient who received six units of red blood cells for a bleeding most likely related to the transcaval access (which stopped without any further intervention). Five more VARC-2-defined major vascular complications without life-threatening bleeding were noted: one patient received two units of red blood cells within the first 48 hours after TAVI due to a gradual haemoglobin drop (most likely access-related, however without evidence of this); one patient received three units of red blood cells and additional sealing of the aortic puncture site with a covered stent (Endurant™ II; Medtronic) one day post TAVI due to a gradual haemoglobin drop of nearly 3 g/dL; one patient experienced a lumbar artery perforation with retroperitoneal haematoma and haemoglobin drop of nearly 5 g/dL and received one unit of red blood cells; and two further patients required vascular surgery within a few hours of TAVI completion because of acute lower limb ischaemia due to an occlusive Angio-Seal™ closure device (Terumo Corp., Tokyo, Japan) that was used to close an 8 Fr femoral arterial access. One of these latter two patients developed sepsis, required dialysis and died 16 days after TAVI due to multiple organ failure. In conclusion, there were overall five VARC-2-defined major vascular complications possibly related to the transcaval access site.
The overall rate of new pacemaker implantation was 9% (4/45), excluding patients with a pre-existing pacemaker. The median length of hospitalisation was four days (IQR 3-7 days); 12 patients were discharged home the day after TAVI.
CLINICAL FOLLOW-UP
Clinical outcome data are shown in Table 2. At 30 days of follow-up, there were no deaths following hospital discharge and no re-hospitalisations for bleeding complications, worsening congestive heart failure or valve-related symptoms. The composite early safety endpoint occurred in 10 patients (20%) and was mostly driven by VARC-2-defined major vascular complications. The clinical efficacy endpoint was reached in 44 patients (88%). There were no cases of clinical valve thrombosis or endocarditis. One patient with multiple cardiovascular risk factors and pre-existing atrial fibrillation presented with an ischaemic stroke at day 50 (with no evidence of valve thrombosis or dysfunction).
Discussion
Although the need for non-TF TAVI procedures has decreased as a result of improved introducer sheaths and smaller transcatheter heart valve delivery systems, there will always remain a select group of patients that can only be treated by alternative access. This study is the first to report on the transcaval TAVI experience in Europe, bearing in mind that this includes the initial experience (first cases) of transcaval TAVI in five different centres.
The outcomes of transcaval TAVI reported in this study demonstrate the feasibility and safety of this approach. Both procedural and short-term clinical outcomes were satisfying, especially considering the high-risk profile of the patient population. Transcaval access was successful in 49/50 patients and device success was obtained in 94%. Half of the patients had a residual aorto-caval shunt upon completion of the TAVI procedure. These shunts, however, had no haemodynamic significance and none of the patients was re-hospitalised for bleeding, transfusion or dyspnoea within 30 days of discharge. Greenbaum and colleagues previously reported that more than 80% of these remaining aorto-caval shunts spontaneously close by 42 days (range 7-189 days)1.
In this patient cohort, rates of VARC-2-defined life-threatening bleeding and major vascular complications possibly related to transcaval access were 4% and 10%, respectively. This is consistent with the outcomes reported for the largest series (n=100) of transcaval TAVI cases in the USA to date, in which rates of life-threatening bleeding and major vascular complications were 7% and 13%, respectively6. These major vascular complication rates are also in line with the TF-TAVI cohorts of the high-risk TAVI trials (PARTNER IA and CoreValve High-Risk), and life-threatening bleeding rates are in line with those reported for the TF-TAVI cohorts in the intermediate-risk TAVI trials (PARTNER 2: TF cohort 7%, transapical cohort 23%; SURTAVI: 12% - considering life-threatening or major bleeding). Importantly, there were no major vascular complications or bleeding events after discharge. It is noteworthy that two major vascular complications in this patient cohort were related to embolisation of an Angio-Seal collagen plug, used to close the arterial access that enabled introduction of the gooseneck snare. Both complications required vascular surgery, and one of the patients died following dialysis and sepsis. A learning point from this observation could be to use suture-based rather than collagen-based closure devices in these patients with severe peripheral vascular disease.
Finally, we would like to emphasise that every patient considered for TAVI should be discussed at the local Heart Team meeting and that transcaval TAVI could be considered if: (1) transfemoral access and transaxillary/subclavian access are not good options, (2) the procedure can be performed in a centre with good experience in different structural heart interventions, (3) a dedicated structural interventional team with the necessary technical skills is available, and (4) the initial transcaval TAVI cases can be guided and supervised by a proctor with experience in transcaval TAVI.
Limitations
This study has all the limitations of a retrospective study and a relatively small sample size (owing to the highly selected patient profile). Furthermore, longer-term follow-up and comparison with other alternative access TAVI techniques were not addressed; however, they were not the remit of this study.
Conclusions
Transcaval access proved to be a feasible and safe alternative TAVI access strategy for high-risk patients with severe aortic stenosis not suitable for a transfemoral and transaxillary/subclavian access. It can be expected that the outcomes of this TAVI approach will continue to improve over time, as centres gain more experience with the technique and a dedicated caval-aortic closure device becomes available.
|
Impact on daily practice Transcaval TAVI proved to be an alternative option for patients deemed unsuitable for any other percutaneous transvascular approach, thereby avoiding the more complication-prone transthoracic access. |
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Conflict of interest statement
O. De Backer has been consultant for and has received institutional research grants from Abbott and Boston Scientific. T. Pilgrim has received research grants to the institution from Boston Scientific, Biotronik and Edwards Lifesciences; speaker fees from Boston Scientific and Biotronik; and is part of a paid clinical event adjudication committee of HighLife SAS. M. Kasel is a proctor and consultant for Edwards Lifesciences and Medtronic. S. Redwood has received speaker fees from Edwards Lifesciences and has served as an international advisory board member for Medtronic. S. Windecker has received research and educational grants to the institution from Abbott, Amgen, Boston Scientific, Biotronik, Bayer, BMS, CSL Behring, Edwards Lifesciences, Medtronic, Polares and Sinomed. A. Greenbaum has served as a proctor for Edwards Lifesciences, Medtronic, and Abbott Vascular and owns equity interest in Transmural Systems. L. Søndergaard has been consultant for and received institutional research grants from Medtronic, Abbott, Boston Scientific and Edwards Lifesciences. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.

