Abstract
The standard approach for transcatheter aortic valve implantation (TAVI) is through the transfemoral retrograde route, because it is minimally invasive and it is feasible under conscious sedation in a totally percutaneous fashion. When the transfemoral access is not feasible, the most used approaches are the transapical for the balloon-expandable Edwards SAPIEN XT valve, the subclavian for the Medtronic self-expandable CoreValve and the transaortic for both prostheses.
We believe that the subclavian approach should be the first option to consider in patients with contraindications to the transfemoral approach, but also in those patients who appear at higher risk of vascular complications in the case of a feasible but difficult transfemoral approach. Although no direct comparison between the subclavian, transaortic and transapical approaches is available, in our opinion the subclavian access should be favoured, because of its lower invasiveness and its feasibility without general anaesthesia. The choice of vascular access should be taken by the Heart Team and should remain patient-centred rather than operator-preference driven.
Introduction
Transcatheter aortic valve implantation (TAVI) has been performed in patients with aortic stenosis who are inoperable or at high risk for surgery for longer than five years with two devices, the balloon-expandable Edwards SAPIEN prosthesis (Edwards Lifesciences, Irvine, CA, USA) and the self-expandable Medtronic CoreValve prosthesis (Medtronic Inc., Minneapolis, MN, USA)1,2. The standard approach for both types of valves developed for TAVI is through the transfemoral retrograde route, because it is minimally invasive and it is feasible under conscious sedation in a totally percutaneous fashion. Although significant technical improvements in sheath diameter and delivery catheter design have been achieved, the transfemoral approach is contraindicated in case of vessel diameter less than 6 mm, in case of severe tortuosity or calcification of the femoral or iliac arteries or of the distal aorta, and in case of previous iliofemoral surgery or stent implantation3-6. In addition, the transfemoral approach should be considered cautiously in patients with an aneurysm of the thoracic or abdominal aorta.
Therefore, alternative routes for TAVI delivery have been developed, including the transapical access for the balloon-expandable Edwards SAPIEN XT valve7, the subclavian access for the Medtronic self-expandable CoreValve8,9 and the transaortic access for both prostheses10,11. Recently, the unconventional carotid artery access has been described for both prostheses12,13.
However, the availability of alternative vascular accesses for TAVI should not modify the principle for which this procedure was conceived, that is the attempt to implant an aortic prosthesis in the least invasive fashion possible. Therefore, the choice of vascular access should remain patient-centred rather than operator-preference driven.
Prognostic impact of lower extremity artery disease
The presence of lower extremity artery disease (LEAD), ranging from 19% to 42% in patients undergoing TAVI14-16, mandates an accurate preoperative assessment, usually performed by means of computed tomography (CT), in order to identify the safest vascular access. Choosing the transfemoral access for TAVI in the presence of severe LEAD increases the risk of periprocedural and postprocedural vascular complications, with a negative impact on clinical outcome14,17. In the Italian CoreValve registry15, the presence of major access site complications was a strong independent predictor of 30-day mortality (OR 8.47, 95% CI: 1.7-43; p=0.01), stronger than depressed left ventricular ejection fraction, prior balloon aortic valvuloplasty and diabetes. Similarly, in the PARTNER trial, patients undergoing transfemoral TAVI with the SAPIEN valve showed a 30-day mortality of 14.1% in case of major vascular complications vs. 3.1% in the absence of such complications (p<0.0001)18. In addition, the presence of LEAD has been identified as an independent predictor of mortality even in patients undergoing TAVI through a surgical, non-transfemoral access (transapical or trans-subclavian)19. In particular, the in-hospital mortality was 15.7% vs. 10.5% (p=0.001) for patients with and without LEAD, respectively. These results suggest a role for LEAD as a marker of concomitant comorbidities, which are effectively responsible for the worse in-hospital outcome in non-transfemoral patients.
Considering these data, the availability of a safer vascular access is clinically important not only when the transfemoral access is not technically feasible, but also when it looks risky because of borderline femoral artery diameter, or of severe ilio-femoral atherosclerosis, calcification or tortuosity.
At present, the most common alternatives to the transfemoral approach are the transapical7 for the balloon-expandable Edwards SAPIEN XT valve, and the subclavian and transaortic access for the Medtronic self-expandable CoreValve20,21.
The subclavian approach
The subclavian approach is currently approved as an alternative to the transfemoral approach for the CoreValve, although very recently a new prosthesis for the transapical access has also been approved. The overall rate of subclavian access in the published registries ranges from 2.6% to 20.0%6,8,9. At the moment, the subclavian route is also being tested for the Edwards SAPIEN XT. We previously reported the early results of the initial 54 patients treated through the subclavian access in the Italian CoreValve registry, demonstrating a good procedural success rate and low in-hospital complication rates, similar to those of the 460 transfemoral patients, in spite of a significantly worse preoperative clinical profile20. Importantly, the trans-subclavian access did not require a significant learning curve in terms of procedural duration and complication rates, allowing for a quick shift from the initial use of general anaesthesia to the current standard of local anaesthesia with mild sedation. In fact, the possibility of performing TAVI under local anaesthesia is a major advantage of the subclavian over the transapical and transaortic accesses considering the risks of general anaesthesia in elderly patients who suffer from multiple comorbidities22. More recently, we described the two-year results of the subclavian approach for TAVI in 141 consecutive patients, compared with 141 propensity-matched patients undergoing transfemoral TAVI8. Outcomes after TAVI through the subclavian access were favourable and very similar to those of TAVI through the transfemoral approach. In addition, vascular and bleeding complications directly related to the 18 Fr arterial access were significantly lower in the subclavian cohort, without paying the price of specific subclavian complications.
A smaller population of 35 patients receiving a CoreValve via a subclavian approach in eight centres of the United Kingdom was compared to 253 CoreValve recipients via a transfemoral approach23. Interestingly, 30-day mortality was 0% in the subclavian cohort vs. 4.7% in the transfemoral cohort (p=0.30), and 30-day major adverse cardiovascular and cerebrovascular events showed a trend favouring the subclavian cohort (2.9% vs. 13.4%; p=0.09).
More recently, the results of the CoreValve ADVANCE study, which is the largest multicentre, prospective, fully monitored TAVI study, confirmed that the subclavian approach is safe and feasible and yields good early outcomes, similar to those of the transfemoral approach despite a higher preoperative risk profile24.
Finally, the European Society of Cardiology (ESC) Sentinel registry of Transcatheter Valve Treatment recently reported the results of TAVI in 4,571 patients in 10 European countries25. Large mortality differences were observed according to the vascular access (transfemoral 5.9%, transapical 12.8%, trans-subclavian and other approaches 9.7%; p<0.01). In particular, the latter approaches (subclavian, transaortic) showed a trend to lower mortality compared with the transapical approach, in the presence of similar preoperative risk profiles.
The “cardiac surgery” approaches: transapical and transaortic
Since the first successful case of transapical TAVI via a left mini-thoracotomy, without the need for cardiopulmonary bypass, this minimally invasive approach has gained popularity among cardiac surgeons performing TAVI26. More recently, the transaortic access was described for both CoreValve and SAPIEN prostheses10,11, and has been adopted increasingly in TAVI candidates with contraindications to the transfemoral access. Both the transapical and transaortic accesses are very familiar to cardiac surgeons and do not need a learning curve such as in the subclavian approach. However, it must be underlined that both require general anaesthesia and are definitely more invasive than the subclavian access. Therefore, although no direct comparison between the three non-transfemoral approaches for TAVI is available, a patient-centred choice should favour the subclavian rather than the transaortic and transapical approaches, even assuming a comparable clinical efficacy.
Concerning the transapical access, although it may be as safe as conventional surgical replacement in high-risk patients7, it is definitely much more invasive than transfemoral TAVI and possibly burdened by a higher mortality, even in very experienced hands, as suggested by observational data5,25,27. The French national TAVI registry reported a significantly lower early and late mortality with the transfemoral approach than with the transapical approach6, and similar findings were recently reported from the European Society of Cardiology (ESC) Sentinel registry of Transcatheter Valve Treatment25. Even when the transapical approach is performed with similar mortality and morbidity compared to the transfemoral approach, it is hampered by longer procedural duration and by specific potential complications, including myocardial tearing, development of apical pseudoaneurysm, and accidental damage to a coronary artery28,29. Although these results suggest that the transapical access should be used only when the other less invasive approaches are not feasible, a review of the literature suggests that it is being used also in patients who do not need a non-transfemoral access (Table 1).
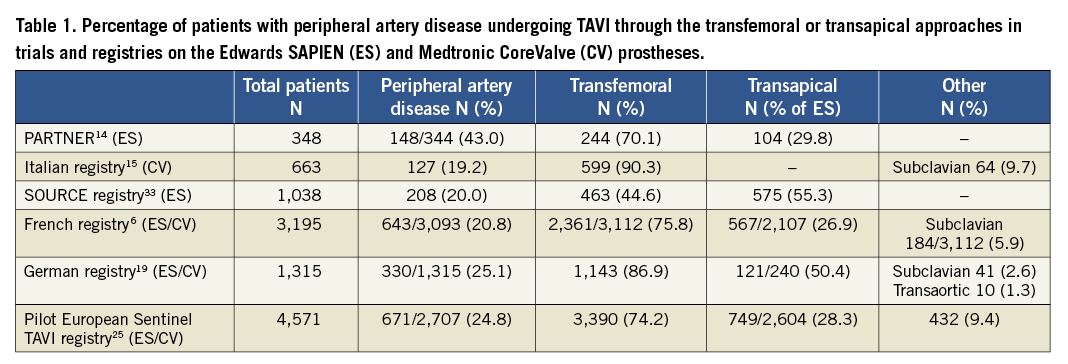
Regarding the transaortic approach, Bruschi et al reported the safety and feasibility of CoreValve implantation through a direct access to the ascending aorta via a right anterior mini-thoracotomy in 25 high-risk patients with unfavourable femoral access21. Importantly, the distance between the aortic annulus and the entry site of the 18 Fr sheath in the ascending aorta must be longer than 6 cm, in order for the CoreValve, which is 5 cm long, to exit completely out of the sheath before deployment. The transaortic approach may also be performed through a mini-sternotomy, which is preferred to mini-thoracotomy by some surgeons and grants a few additional centimetres of space between the entry point of the 18 Fr sheath and the aortic annulus10.
The transaortic approach has important advantages over the transapical approach, primarily avoidance of damage to the left ventricular apex. However, the transaortic approach shares an important limitation with the transaortic approach, i.e., the need for general anaesthesia, and has a specific limitation in the presence of a “porcelain” aorta, with diffuse calcifications preventing a safe puncture of the aortic wall.
Compared with the transfemoral and subclavian approaches, the transaortic access is characterised by a shorter distance between the tip of the 18 Fr sheath and the aortic valve, allowing for a more stable position during valve deployment, particularly important for the correct positioning of the CoreValve prosthesis. On the other hand, the small distance between the tip of the 18 Fr sheath and the aortic annulus does not allow the TAVI prosthesis to align itself to the axis of the aortic valve root when the latter has a “horizontal” configuration (Figure 1 and Figure 2). Consequently, the axis of the prosthesis may not be perpendicular to the plane of the aortic annulus before deployment, making proper positioning of the prosthesis more difficult. This may represent a disadvantage with regard to the left subclavian approach, which allows the prosthesis to conform better to the curvature of the ascending aorta (Figure 1 and Figure 2).
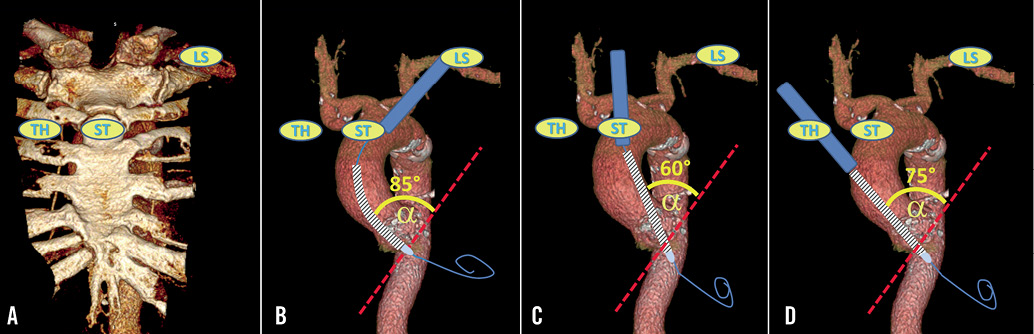
Figure 1. Anteroposterior view of a 3-D reconstruction of a computed tomography of the thoracic aorta in a patient with “horizontal” aortic root. Drawings represent the 18 Fr sheath (blue) used to introduce the CoreValve prosthesis (striped) in the ascending aorta; the red dashed line represents the plane of the aortic annulus. A) Scheme of the access sites for the left subclavian approach (LS), and for the transaortic approach through a mini-sternotomy (ST), and through a mini-thoracotomy in the second left intercostal space (TH). B), C), and D), schemes of CoreValve positioning from the LS, ST and TH approaches, respectively, showing that the LS approach allows the CoreValve to enter the native aortic valve with the most perpendicular alignment with the plane of the aortic annulus (largest α angle).
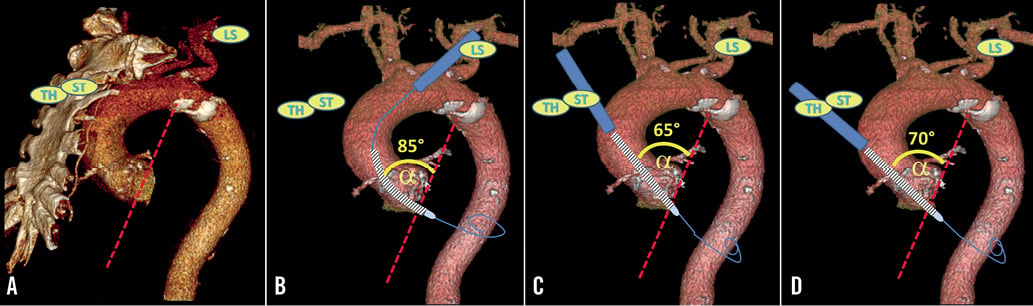
Figure 2. Left anterior oblique view of the same 3-D reconstruction of a computed tomography of the thoracic aorta as in Figure 1. A), B), C), and D), the same as in Figure 1, but for the left anterior oblique view.
In our opinion, the transaortic access should be considered as a third choice for vascular access in TAVI patients, after ruling out the feasibility of both the transfemoral and subclavian approaches, but still preferable to the transapical approach (Figure 3).
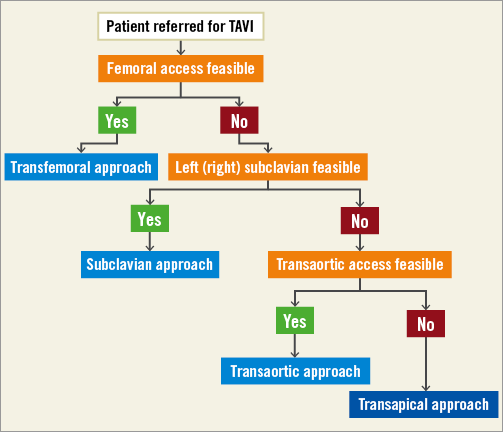
Figure 3. Proposed algorithm for the choice of vascular access for TAVI.
In summary, the familiarity of cardiac surgeons with the transapical and transaortic accesses should not change a patient-centred choice into an operator-preference choice.
Technique of the subclavian access
The subclavian approach requires a cardiac/vascular surgeon to isolate and prepare the subclavian artery for sheath insertion (the usual puncture site is actually just distal to the outer border of the first rib, i.e., where the subclavian artery has changed name to the axillary artery), using standard surgical technique. Local anaesthesia with lidocaine and naropine in combination with mild systemic sedative/analgesic treatment should be used whenever possible, rather than general anaesthesia. The artery can be punctured with the Seldinger technique in the middle of a double purse-string suture, although some surgeons may prefer performing an arteriotomy or placing a graft conduit. The standard 18 Fr sheath used for the transfemoral approach is then advanced over a stiff guide wire through the subclavian artery into the aortic arch and ascending aorta, stopping just below the origin of the brachiocephalic artery. From this point onward, subclavian TAVI is performed with the same technique used for the transfemoral approach. At the end of the procedure, haemostasis is achieved simply by tightening the purse-string sutures and the skin layers are closed in the usual fashion. A drainage tube is rarely needed. Patients should be on aspirin (100 mg/day) before the procedure and indefinitely afterwards; clopidogrel (300 mg loading dose) should be administered before the procedure and continued (75 mg/day) for three months. During TAVI, the patient receives weight-adjusted intravenous heparin to achieve an activated clotting time of 200 to 250 seconds throughout the procedure.
The insertion of an 18 Fr sheath in the subclavian artery may appear alarming, as it is often considered more fragile than the common femoral artery, and its anatomical location is unfavourable in case of vessel rupture. Moreover, the subclavian artery is quite tortuous, particularly in very elderly subjects, and may present focal stenosis or calcifications at its origin. Nevertheless, a thorough preoperative assessment of the anatomy of the subclavian artery, in terms of vessel diameter, degree of tortuosity, presence and extent of calcifications at the origin, together with a careful surgical technique allow for a safe use of this artery for TAVI, as demonstrated by a very wide international experience8,9,30. A specific condition peculiar to the left subclavian access for TAVI is the presence of a patent left internal mammary artery (LIMA) graft to the left anterior descending coronary artery; in fact, positioning an almost occlusive 18 Fr sheath in front of the origin of the LIMA may cause myocardial ischaemia31. To prevent such an occurrence, the subclavian artery diameter should be at least 7 mm, free of atherosclerotic disease, especially proximal to or at the ostium of the LIMA, and with minimal tortuosity at the origin of the LIMA. An injection of dye can confirm a good antegrade flow in the LIMA. To minimise the potential limitation in LIMA flow during TAVI, the 18 Fr sheath can be withdrawn distal to the origin of the LIMA immediately after the advancement of the CoreValve prosthesis across the aortic valve. Our experience in this subset of patients confirms the safety of CoreValve implantation through the left subclavian artery, with no case of periprocedural myocardial ischaemia8,20. The left or right subclavian access is feasible also in patients with a permanent pacemaker in the ipsilateral pectoral region; in fact, the surgical cutdown access to the subclavian artery is usually medial enough to the pacemaker pocket not to interfere with the pacemaker generator and wires.
The left subclavian artery is preferable to the right subclavian artery as an access for TAVI, because it allows for a more coaxial orientation of the CoreValve with the aortic root and annulus. However, the right subclavian access may also be feasible in selected cases with a favourable anatomic configuration with procedural results similar to those of the left subclavian access, as recently reported by Testa et al32. In this study, the subclavian access was chosen in 70 out of 300 patients (23%), with the right side being chosen in 14% of the cases. However, the right subclavian access cannot be considered as standard an approach as the left subclavian access, as it comprises additional technical challenges. Firstly, when coming from the right subclavian artery, the 18 Fr sheath should remain distal to the origin of the right common carotid artery, not to hinder flow to the brain. Secondly, the angle between the delivery catheter and the axis of the ascending aorta is usually wider than from the left subclavian access, making prosthesis implantation more difficult. Therefore, we suggest that the right subclavian access should be performed only when the left subclavian access is not feasible and the orientation of the ascending aorta is not “horizontal”, that is, forming an angle with the horizontal plane of no less than 30°.
Compared with the transfemoral approach, the subclavian access has the advantages of providing a less remote access to the aortic valve. The valve delivery catheter covers a shorter distance, and avoids bending in the tortuosities of the iliofemoral axis and of the thoracoabdominal aorta, with the potential to improve the control of the prosthesis during deployment. Therefore, the subclavian approach may theoretically allow for a more accurate device positioning, reducing the incidence of paravalvular leak and the development of complete heart block requiring permanent pacemaker implantation. Although in our initial report on the subclavian access we observed a trend to a more precise CoreValve positioning20, this was not confirmed in the larger cohort described subsequently8. More recently, the ADVANCE registry also reported a trend towards a reduction in new pacemaker implantation with the subclavian approach24.
Conclusion
We believe that the subclavian approach should be the first option to consider in patients with contraindications to the transfemoral approach, but also in those patients who appear at higher risk of vascular complications in case of a feasible but difficult transfemoral approach. Although no direct comparison between the subclavian, transaortic and transapical approaches is available, in our opinion the subclavian access should be favoured, because of its lower invasiveness and its feasibility without general anaesthesia (too risky for some TAVI patients). The choice of vascular access should be taken by the Heart Team and remain patient-centred.
Conflict of interest statement
A.S. Petronio is a clinical proctor for Medtronic. The other authors have no conflicts of interest to declare.

