
Abstract
The transfemoral (TF) route constitutes the undisputed default access for transcatheter aortic valve implantation (TAVI). In patients in whom anatomical constraints preclude a TF approach, several alternative access routes have been used. Transthoracic (transapical and transaortic) access routes show higher mortality and bleeding complication rates than the TF approach, which is attributable to the higher baseline risk of the selected patients and the more invasive nature of these procedures. Alternative transarterial approaches (transaxillary, transcarotid, transinnominate) have demonstrated high technical success rates and a favourable safety profile in selected patients and are particularly valuable in the presence of poor respiratory function or previous cardiothoracic surgery. The transcaval approach is an innovative fully percutaneous approach that allows the introduction of large-bore sheaths and shows promising results in high-risk patients. Diligent procedural planning, appropriate patient selection and the expertise of the Heart Team allow the achievement of an adequate safety and efficacy profile of TAVI performed via alternative access. Future studies incorporating standardised and independent outcome assessment are required to gain further knowledge on the risk/benefit relation pertaining to the specific approaches and improve selection of the appropriate access route for the individual patient.
Abbreviations
MSCT: multislice computed tomography
SAVR: surgical aortic valve replacement
STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality
TA: transapical
TAo: transaortic
TAVI: transcatheter aortic valve implantation
TAx: transaxillary
TC: transcarotid
TCv: transcaval
TF: transfemoral
TI: transinnominate
Introduction
In 2002 the first transcatheter aortic valve implantation (TAVI) was performed via an anterograde transseptal approach1. Since then, TAVI has become an established therapy for patients with severe symptomatic aortic stenosis (AS) deemed at increased risk for surgical aortic valve replacement (SAVR)2.
Vascular complications and access-site bleeding remain the most frequent complications of TAVI (Supplementary Table 1, Supplementary Table 2)3. They are associated with increased mortality, longer hospital stay and higher costs of care4,5. The frequency of bleeding complications drastically declined following the introduction of low-profile delivery systems and introducer sheaths, widespread adoption of the transfemoral (TF) access route and closure device systems, as well as a shift in the risk profile of the treated population3,6-8. Meta-analyses of randomised controlled trials comparing TAVI to SAVR in high- and intermediate-risk patients indicate a survival benefit of TF TAVI compared to SAVR9. Due to the familiarity of the interventional cardiologist with the femoral access, improved delivery systems allowing insertion in small-calibre and diseased iliofemoral arteries and the compelling scientific evidence showing favourable, highly reproducible results, the TF route has nowadays become the undisputed default access approach for TAVI2. Nevertheless, some patients cannot be subjected to TF access due to small arterial vessel calibres, calcifications, tortuosity, or a combination of these factors. Several alternative access sites have been developed, used, and technically refined in order to enable minimally invasive transcatheter treatment of aortic stenosis when the anatomic properties of the iliofemoral arteries are unfavourable (Figure 1). This review aims to provide an overview on the use and state of evidence on the different alternative access routes used for TAVI.
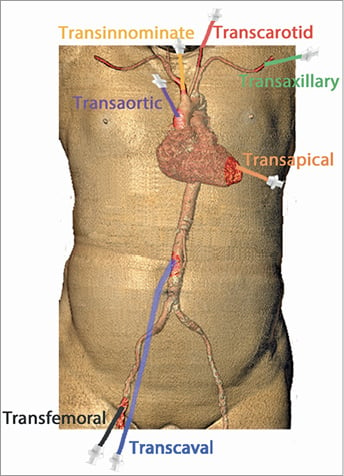
Figure 1. Access routes for TAVI.
EVOLUTION OF TAVI SYSTEMS
TAVI systems have undergone constant refinements to address limitations such as paravalvular regurgitation, stroke, vascular complications and pacemaker rates. Notably, a remarkable reduction in introducer sheath size has been achieved (Supplementary Table 3).
In less than 10% of patients undergoing TAVI, the iliofemoral arteries do not formally accommodate the latest low-profile delivery systems10. The low-profile self-expanding valve systems, notably the CoreValve® series (Medtronic, Minneapolis, MN, USA), are most frequently applied for peripheral arterial alternative access, occasionally in a sheathless fashion. When considering the eSheath for an alternative peripheral arterial access, the maximum local expansion (Supplementary Table 3), as well as the fact that the crimped valve needs to be aligned onto the balloon within the body, has to be taken into account.
ADOPTION OF ACCESS ROUTES
A clear rise in the proportion of patients treated via the TF access in recent years reflects the favourable outcomes and technical advances of the default TAVI access route. In large registries from Europe and the USA, the proportion of patients treated by the TF approach has consistently increased over the years, reaching more than 85% in 2015 (Figure 2)7,8,11-14. Nowadays, many individual centres reach TF rates of well above 90%.
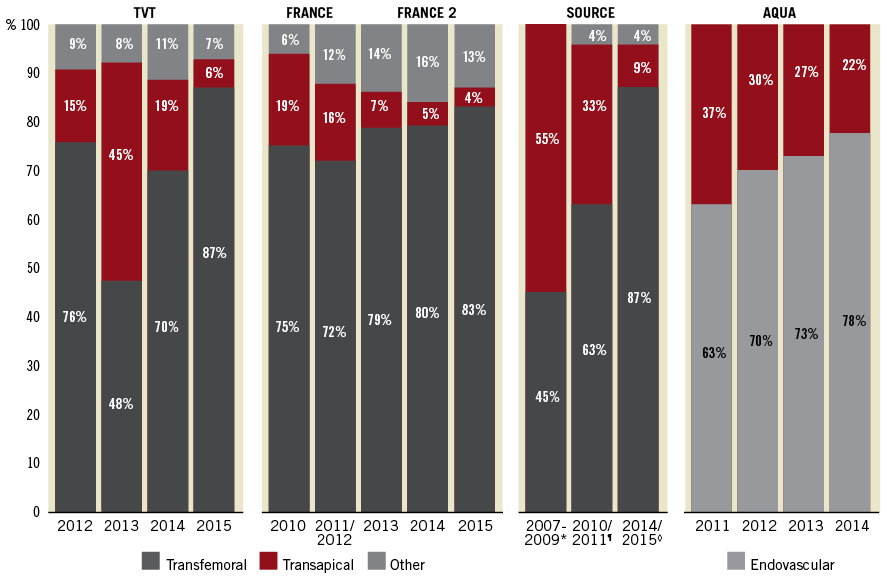
Figure 2. Temporal trends of access site use for transcatheter aortic valve implantation (TAVI). The proportion of transfemoral, transapical and other access routes used for TAVI in the TVT8, FRANCE11 and SOURCE (*Source12, ¶Source XT13, ◊Source 37) registries as well as endovascular access and transapical use reported in the AQUA registry14.
In the following paragraphs, the different access routes are presented in anatomical order from central to peripheral (Figure 1).
Transapical (TA)
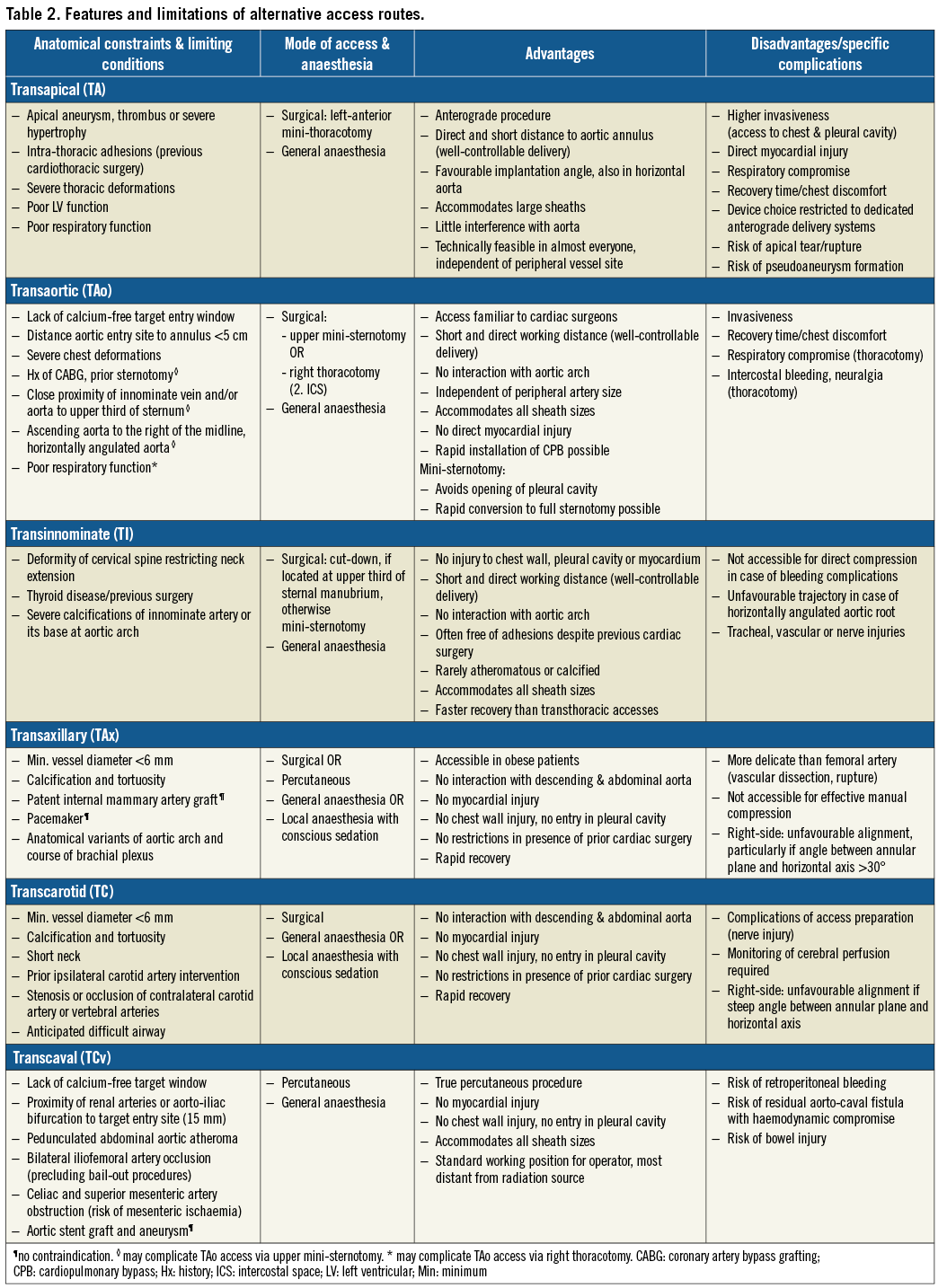
The first case of TA TAVI without cardiopulmonary bypass was performed in 200515. During the early years of TAVI, the TA approach rapidly emerged as the most frequently used alternative access route as the large introducer sheaths precluded alternative peripheral arterial access routes for patients with unsuitable iliofemoral arteries16-18. More recently, its use has clearly declined owing to the high proportion of patients amenable to a TF approach, complications related to the TA access site, and the advent of a variety of alternative access strategies (Figure 2).
Multislice computed tomography (MSCT) allows assessment of the relationship and distance of the apex to the chest wall and the angle of trajectory from the apex to the aortic annulus. Currently available transapical TAVI systems are limited to the balloon-expandable SAPIEN 3 (Edwards Lifesciences, Irvine, CA, USA) delivered over an 18 or 21 Fr sheath and the self-expanding ACURATE neo™ (Boston Scientific, Marlborough, MA, USA) with an outer transapical delivery system diameter equivalent to a 19 Fr sheath (Supplementary Table 3). The steps of the TA access have been published elsewhere19.
EVIDENCE
Studies reporting independently adjudicated adverse events after TA TAVI are listed in Table 1. A meta-analysis, incorporating the landmark PARTNER 1 and PARTNER 2A trials, showed no survival benefit of transthoracic TAVI (TA or transaortic [TAo]) over SAVR at two years (hazard ratio [HR] for all-cause mortality 1.17, 95% CI: 0.88-1.56)9, nor did the results of the PARTNER 1A trial when comparing five-year mortality rates between TA TAVI and SAVR (HR 1.37, 95% CI: 0.98-1.92)20.
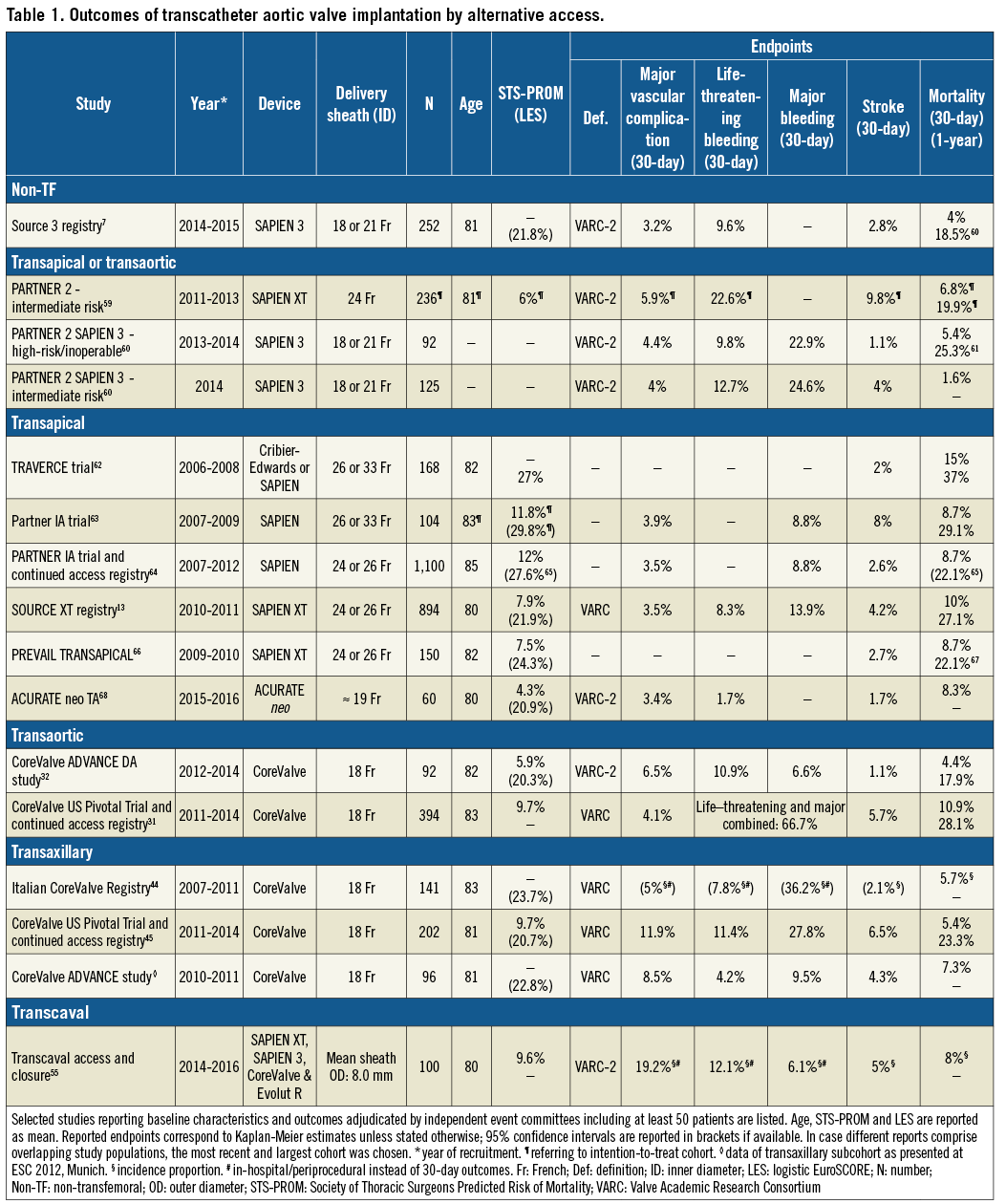
Observational studies and registries report higher mortality rates in patients treated by TA compared to TF TAVI, but inferences are precluded due to differences in baseline risk profiles13,18,21. Propensity score-matched or score-adjusted comparisons between TA and TF TAVI derived from studies using independent event adjudication suggest a higher short- and long-term mortality, similar 30-day stroke rates, higher rates of major bleeding and longer length of hospital stay for patients treated by the TA approach. Blackstone et al compared 501 propensity-matched pairs of high-risk or inoperable patients with severe AS treated with TA or TF TAVI in the PARTNER 1 trial or continued access registry16. TA compared to TF TAVI was associated with a significantly higher 30-day mortality rate (9.1% versus 3.7%), a similar 30-day stroke rate (3.4% vs. 3.3%), a longer length of hospital stay (5 versus 8 days; p<0.0001), and a trend towards a lower rate of procedural major vascular injury (3.8% vs. 6%, p=0.10) but a higher rate of major bleeding (7.2% vs. 4.8%, p=0.12)16. A propensity score-adjusted study derived from the same PARTNER 1 trial and registry cohort including 2,084 patients confirmed a negative impact of the TA approach on two-year all-cause mortality for both patients with (left ventricular ejection fraction [LVEF] <50%) and those without left ventricular dysfunction and reported a lower degree of LVEF recovery in the TA group (TF-TA difference +4.04%, 95% CI: 2.39% to 5.69%; p<0.0001) compared to TF TAVI22. Some risk-adjusted and risk-unadjusted comparisons showed no difference in mortality, stroke or bleeding complications between TA and TF access; however, these studies were smaller and lacked independent event adjudication23,24. Adjusted comparisons between TA and other alternative approaches that allow meaningful inferences are not available25-27.
Transaortic (TAo)
The first TAo TAVI was reported in 200928. Rates of TAVI performed by TAo access have increased, partially at the expense of the TA approach, as the former entails less respiratory compromise and no direct myocardial injury, while preserving the benefit of a short distance to the landing zone and a good control of valve delivery (Table 2)29. A suitable calcium-free entry site at the anterolateral ascending aorta with a minimum distance of 5 cm (6 cm for the CoreValve series) to the aortic annular plane and a favourable trajectory to the landing zone are preconditions for TAo TAVI. Further assessments derived from MSCT focus on the course and angulation of the aorta and innominate vein as well as the localisation of aorto-coronary bypass grafts if present. A mini-sternotomy is favoured in case of respiratory compromise to avoid entering the pleural cavity29. It also allows rapid conversion to full sternotomy if needed. Recovery is faster than after thoracotomy with rib spreading29. A right anterior mini-thoracotomy is more suitable if the aorta is located to the right of the midline; it prevents injury to bypass grafts, if present, and provides a more coaxial trajectory to the annular plane in case of a horizontal angulation of the aortic root. Procedural steps of TAo TAVI have been published previously30.
EVIDENCE
Studies based on independently adjudicated outcomes after TAo TAVI are scarce (Table 1)31,32. In 92 patients undergoing TAo TAVI in the CoreValve ADVANCE Direct Aortic study, all-cause mortality was 4.4% and the stroke rate was 1.1% at 30 days32. Thirty-day rates of major vascular complications, life-threatening or disabling bleeding and major bleeding were 6.5%, 10.9% and 6.6%, respectively. Notably, only 30% of the included patients were not considered candidates for a TF or other alternative approaches31. The “Registry of the Utilisation of the TAo-TAVR approach using the Edwards SAPIEN Valve” (ROUTE) reported the outcomes of 301 patients (mean age 81.7 years, mean Society of Thoracic Surgeons Predicted Risk of Mortality [STS-PROM] score 9.0%), but did not include independent event adjudication33. Valve success was documented in 96.7%, 30-day mortality amounted to 6.1%, stroke to 1.0%, major vascular complications to 3.4%, and life-threatening bleeding to 3.4%33.
A comparison between TAo and TF access in 394 propensity score-matched pairs of the CoreValve US Pivotal Trials and continued access registry showed a significantly higher rate of 30-day all-cause mortality (10.9% vs. 4.1%, p<0.001), stroke (5.7% vs. 2.6%, p=0.028) and life-threatening or major bleeding (66.7% vs. 35.4%, p<0.001) for the TAo group, but a lower rate of major vascular complications (4.1% vs. 9.4%, p=0.003)31. Observational studies report similar survival rates between TAo and TA TAVI; however, well-adjusted comparisons and independent event adjudication are lacking25,26.
Transinnominate (TI)
The first TI TAVI reported in the literature was conducted in 201134. The transinnominate (or suprasternal) approach is reported to have few anatomical constraints as the innominate artery usually provides a generous luminal diameter, stays unaffected in patients with previous cardiac surgery, and only rarely shows relevant atheromatous disease (Table 2)35.
Preparatory MSCT assessment focuses on the relationship and angle of the sternum to the spine, the course of the innominate artery in relation to the sternum, the diameter, extent and location of calcium, and the anticipated trajectory to the annular plane35. In order to avoid cerebral hypoperfusion after sheath insertion, perfusion can be surveilled by means of a right radial arterial line or cerebral oximetry36. Procedural steps have been published elsewhere35.
EVIDENCE
Only small observational studies without independent event adjudication have reported on outcomes after TI TAVI. The largest one comprised 30 patients (mean age 79.8 years, mean STS-PROM score 6.7%) and reported no relevant adverse events during hospitalisation and a short median hospital duration of four days37. In another series encompassing 26 patients, all patients survived to 30 days, one stroke occurred and there were three major vascular access-site related complications36.
Transaxillary (TAx)
The first reports on TAVI via a TAx approach were published in 2008 and 200938,39. The frequently applied term “transsubclavian access” is misleading as generally the axillary artery is used as entry site. Cardiac surgeons are familiar with this access as it is occasionally used as a cannulation site for cardiopulmonary bypass. The axillary arteries often show little calcification and tortuosity as well as larger lumina, even in the presence of diseased iliofemoral arteries40. The short distance to the valve landing zone allows good control of the delivery system. TAx TAVI can be performed as a true percutaneous procedure and under local anaesthesia with conscious sedation41,42. Particular care has to be taken as the axillary arteries are more delicate than the iliofemoral ones and effective manual compression is hampered by the anatomical conditions42.
Information on the origin and course of the subclavian and axillary arteries, their diameter and tortuosity, as well as the presence, localisation and extent of calcification is obtained by MSCT. The left axillary artery is generally preferred over the right one due to a more favourable implantation angle providing better axial alignment of the device with the aortic root. An angle >30° between the annular plane and the horizontal axis is regarded as a significant limitation for right-sided TAx access42. The artery’s minimum diameter should measure ≥6 mm. Specific conditions such as a patent LIMA graft or a pacemaker are not strict contraindications, but have to be taken into account when evaluating patients for a TAx approach42. In the former case, verification of a sufficiently large vessel diameter (at least 7-8 mm) is recommended to ensure perfusion of the graft during the intervention42. Procedural steps have been published in detail previously42,43.
EVIDENCE
The majority of the experience with this access route is based on the use of the self-expanding CoreValve platform. Three studies have reported outcomes based on independent event adjudication (Table 1). A propensity-matched comparison of 141 pairs treated either by TAx or TF access showed similar adverse event rates in the two groups and a lower rate of direct access-related bleeding complications with the TAx approach44. Gleason et al matched 202 patients from the CoreValve US Pivotal Trial and continued access registry treated by TAx approach 1:1 to patients undergoing TF TAVI based on propensity scores45. Mortality amounted to 5.4% at 30 days, and 23.3% at one year (Table 1). Rates of major vascular complications and life-threatening bleeding were 11.9% and 11.4%, respectively45. Neither the mortality rates nor rates of other adverse events differed significantly between the TAx and TF groups45. Ninety-six patients treated by TAx TAVI in the CoreValve ADVANCE study showed a higher incidence of major adverse cardiovascular and cerebrovascular events (13.5% vs. 7.9%, p=0.05) compared to patients treated by TF TAVI, but they also had a higher mean logistic EuroSCORE (data presented at ESC 2012, Munich). For individual endpoints, differences in 30-day rates did not reach statistical significance.
A meta-analysis encompassing 618 patients treated by TAx and 3,886 patients treated by TF TAVI reported no difference in 30-day mortality, stroke, major vascular complications or life-threatening bleeding despite a higher mean logistic EuroSCORE and a higher prevalence of coronary and peripheral artery disease in the TAx group46. Compared with transthoracic (TA and TAo) access routes, a trend towards fewer periprocedural events with the TAx approach is suggested by registry data; however, populations differed in baseline characteristics and risk profiles27,47.
Several studies have proved the feasibility and suggest the safety of a direct percutaneous TAx approach41,48. The largest one included 100 patients in whom access vessel closure was performed with two Perclose ProGlide® systems (Abbott Vascular) achieving successful closure in 94.8%; 11% required covered stent implantation, but no major access-site complication occurred. Thirty-day mortality amounted to 6% and life-threatening bleeding to 3%; no strokes were reported41.
Of note, nerve injury as a consequence of the TAx approach appears to occur rarely49.
Transcarotid (TC)
The first TC TAVI procedure was performed in 200950. Cardiovascular surgeons are familiar with the TC access. The short distance to the annulus provides good control of the valve delivery system. Coaxial alignment of the system and the aortic root is generally better from the left carotid artery. MSCT allows assessment of vessel properties and identification of congenital variants of the aortic arch. Flow-limiting stenoses can be detected by Doppler ultrasound examination. Magnetic resonance imaging (MRI) with cerebral angiography is used to assess the arterial circle of Willis and collateral blood flow and to detect previous strokes51. At the beginning of the procedure, cerebral perfusion is assessed during a cross-clamping test by means of a neurological assessment if performed under local anaesthesia, or by use of cerebral oximetry and distal backflow arterial pressure monitoring if the patient is under general anaesthesia. In case of signs of hypoperfusion, a femoro-carotid shunt is inserted. Procedural steps have been published previously51,52.
EVIDENCE
Three large prospective observational reports from French TC TAVI cohorts are available; however, none of these reported independently adjudicated events51-53. The largest one included 174 patients (mean age 80.5 years, mean STS-PROM score 8.4%)53. Vascular access and valve deployment was successful in all cases. All-cause mortality amounted to 7.4% at 30 days and 12.6% at one year53. A higher rate of perioperative strokes was observed with general anaesthesia (four strokes [2.2%] and six transient ischaemic attacks [TIA] [3.4%]) than under local anaesthesia with conscious sedation (none)53. The majority of the events were localised contralateral to the access site and hence appear to be related to the TAVI procedure rather than the access. No major vascular complication related to the carotid access occurred. Another study with 145 patients (mean age 79.8 years, mean logistic EuroSCORE 20.7%) reported similar 30-day mortality (6.3%), stroke (2%) and TIA (3.5%) rates52. One case of late asymptomatic carotid dissection was recorded52. Bleeding complications were frequent (25.6%) but mainly driven by a liberal use of blood transfusions52.
Transcaval (TCv)
The first human case of TCv TAVI was performed in 201354. The transcaval approach relates to obtaining percutaneous femoral venous access and entering the aorta through the inferior vena cava by means of an electrified stiff coronary guidewire. Subsequently, microcatheters in a “mother and child” set-up, a stiff guidewire and, eventually, the delivery sheath are inserted. At the end of the procedure a nitinol occluder device is implanted at the aortic entry site.
Rapid pressurisation of the retroperitoneal space and consecutive drainage of aortic blood via shunting into the vena cava constitutes the concept underlying the feasibility of this approach and explains haemodynamic stability as observed during temporal removal of the cavo-aortic sheath or in case of persistent aorto-caval fistula55,56.
Neither surgeons nor interventional cardiologists were familiar with this approach prior to its introduction for TAVI. However, it is performed by use of off-the-shelf equipment and procedural steps can be acquired rapidly with the support of proctors. The TCv route constitutes the only non-transthoracic approach that does not rely on an adequately sized peripheral artery for delivery sheath insertion. The distensible venous system allows accommodation of large sheaths with no restriction regarding the choice of valve system.
MSCT is crucial for assessment of eligibility for TCv TAVI. A sufficiently large calcium-free target zone (≥1 cm is favourable) of the right-sided abdominal aortic wall and a trajectory from the vena cava without interposed obstacles (bowel) are preconditions57. Projections orthogonal to the anticipated cavo-aortic crossing trajectory, as well as anatomical landmarks used for orientation during fluoroscopy, are identified. At least one peripheral artery should be patent and provide a lumen that allows bail-out aortic balloon occlusion or covered stent implantation. The aortic entry site should be sufficiently distant from the major arterial branches and the aorto-iliac bifurcation (≥1.5 cm) to avoid occlusion of relevant arteries in case of bail-out stent implantation. The distance between the femoral venous puncture and aortic entry site provides information on the adequacy of the delivery sheath working length57. Of note, procedures across abdominal grafts as well as abdominal aortic aneurysms are feasible57. Step-by-step instructions have been published previously56. Moving image 1 illustrates TCv access and closure.
EVIDENCE
A prospective observational study including 100 patients and comprising on-site monitoring, independent event adjudication as well as core laboratory assessment of MSCTs was conducted between July 2014 and June 201655. Patients were at extreme risk or considered unsuitable for TF, TA or TAo access and hence showed a high mean STS score of 9.7% and a high burden of comorbidities55. Device success, defined as successful transcaval access and deployment of a closure device without death or emergency open abdominal surgery, was achieved in 98% of the patients. No patient died as a direct consequence of TCv access or required surgical bail-out. The observed 30-day mortality rate amounted to 8%, and the stroke rate to 5%55. Rates of major vascular complications, life-threatening bleeding and major bleeding were 19.2%, 12.1%, and 6.1%, respectively55. Notably, a considerable proportion of major bleeding events could be attributed to retroperitoneal haematoma, not clinically apparent but detected in the routinely performed pre-discharge MSCTs.
In 36% of the patients, the aorto-caval fistula was closed at the end of the procedure55. Abdominal aortic covered stent implantation was performed in 8% of the patients. There were no vascular complications after hospital discharge. In 72% of the patients for whom a MSCT was available, no aorto-caval fistula was present at 30 days, and for 64% of the overall study population imaging proof of the occluded aorto-caval tract was available55.
Discussion
Since the advent of TAVI a variety of alternative access modes has been used and described. However, studies encompassing independent adjudication of events after TAVI performed via alternative access are scarce and completely lacking for the TC and TI approaches. Information and publication bias possibly distort the knowledge gained from observational studies.
Selection of an alternative access route is inherently linked to the presence of extensive peripheral artery disease and a higher burden of comorbidities; unsurprisingly, non-transfemoral TAVI inevitably comes at the price of increased adverse event rates driven by the baseline risk of these patients. Comparisons between different alternative access routes derived from observational studies are hampered by selection bias and residual unmeasured confounding. Randomised head-to-head comparisons between alternative access routes are precluded as patients selected for different approaches are not interchangeable, and adequately powered trials are not realisable due to the low rates of alternative access use.
The transthoracic approaches (TA and TAo) constitute the alternative approaches for TAVI that are best documented with respect to evidence derived from studies comprising independent event adjudication. Propensity-matched comparisons and observational data suggest a higher mortality rate, more bleeding complications and a prolonged recovery compared to the TF approach. Although selection bias and residual confounding probably distort these comparisons, the findings are consistent and plausible in view of the higher invasiveness of the transthoracic procedures comprising myocardial injury (in TA) and respiratory compromise.
The arterial alternative access modes (TAx, TC, TI with cut-down) spare opening of the chest. They are valuable alternative access options, in particular in patients with chest pathologies, poor respiratory function or previous cardiothoracic surgery. Studies reporting independently adjudicated events are scarce and limited to the TAx approach. Available observational reports on TAx TAVI suggest a high technical success rate, a favourable safety profile and reproducible results if conducted in appropriately selected patients.
The transcaval access constitutes an innovative approach performed completely percutaneously and allowing the introduction of large-bore sheaths despite the presence of severe peripheral artery disease. Results of a well-conducted prospective study have dispelled concerns related to access complications and uncontrollable bleeding and showed acceptable adverse event rates considering the high-risk features of the patients included55. Dedicated devices for aortic entry site closure will probably render this approach more attractive and increase its adoption in clinical practice.
Due to the unavailability of comparative data, the choice of alternative access should be made by the Heart Team on a case-by-case basis. Each alternative approach is associated with advantages, disadvantages and specific complications (Table 2). Imaging by means of contrast-enhanced MSCT is critical to assess patient eligibility for different access sites, to anticipate complications and to plan bail-out strategies. In general, the least invasive method should be chosen in order to minimise post-procedural pain and allow fast recovery, particularly in elderly and frail patients. However, next to anatomical preconditions and comorbidities, the choice of access is largely driven by the operator and local site experience and preference, as well as the infrastructure and reimbursement. Given the relatively low volume of TAVI patients requiring alternative access, the learning curve leading to technical proficiency can take significant time and smaller centres may concentrate on a specific alternative approach.
Conclusion
We conclude that with appropriate patient selection and an experienced team a favourable safety and efficacy profile can be achieved for most alternative approaches for TAVI. Future studies with thorough and standardised outcome assessment will shed further light on the risk/benefit relation pertaining to different approaches and improve selection of the appropriate access route for the individual patient.
Conflict of interest statement
A. Greenbaum is a proctor for Edwards Lifesciences and Medtronic and has pending patents on equipment related to the transcaval access and closure that have been assigned to his former employer, the Henry Ford Hospital, Detroit, USA. T. Pilgrim has received research grants to the institution from Biotronik, Symetis, and Edwards Lifesciences, and speaker fees from Boston Scientific. G. Tarantini has received research grants to the institution from Cordis and Boston Scientific, and speaker fees from Abbott, Medtronic, Boston Scientific, and Edwards Lifesciences. S. Windecker has received research grants to the institution from Abbott, Amgen, Biotronik, Boston Scientific, and St. Jude Medical. J. Lanz has no conflicts of interest to declare.
Supplementary data
Supplementary Table 1. Valve Academic Research Consortium-2 (VARC-2) definitions of vascular access-site and access-related complications and bleeding complications69.
Supplementary Table 2. Outcomes of transcatheter aortic valve implantation by transfemoral access.
Supplementary Table 3. Currently available transcatheter aortic valve implantation systems and predecessors with CE (Conformité Européenne) mark.
Moving image 1. Transcaval access and closure.
To read the full content of this article, please download the PDF.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Transcaval access and closure.

