Abstract
Aims: We aimed to describe the first percutaneous transcaval TAVI in Europe using the expandable introducer sheath (eSheath; Edwards Lifesciences, Irvine, CA, USA) for implantation of an Edwards SAPIEN 3 aortic valve (23 mm).
Methods and results: A 72-year-old male patient presented with dyspnoea (NYHA III) in part due to severe aortic stenosis. Concomitant diseases were severe peripheral artery disease, severe chronic obstructive pulmonary disease (GOLD IV) and renal insufficiency, such that neither a transfemoral nor a transapical approach was considered favourable. An eSheath was introduced into the abdominal aorta via the femoral vein and the inferior vena cava. TAVI was then performed according to standard procedures. After TAVI, the veno-arterial junction was occluded with a 6 mm AMPLATZER Muscular VSD Occluder (St. Jude Medical, St. Paul, MN, USA) using an 8.5 Fr Agilis™ sheath (St. Jude Medical) inside the TAVI sheath. Aortography showed no retroperitoneal accumulation of contrast media. The femoral vein access site was closed using two prepositioned sutures (ProGlide; Abbott Vascular, Santa Clara, CA, USA). A CT scan the following day showed no retroperitoneal haemorrhage.
Conclusions: Percutaneous transcaval venous access to the aorta may provide a new access strategy for TAVI in otherwise ineligible patients and appears safe with expandable sheath technology.
Introduction
Transcatheter aortic valve implantation (TAVI) has emerged as an alternative treatment for patients with severe aortic stenosis and at high surgical risk or for inoperable patients. If the vascular anatomy does not allow a transfemoral approach or if surgical access such as transapical, transaortic or subclavian implantation is not desirable, the transcaval approach has recently been described as a potential alternative1.
Case report
A 72-year-old man was referred to our hospital with severe symptomatic aortic stenosis (aortic valve area 0.88 cm2, mean aortic valve gradient 47 mmHg). He suffered from dyspnoea (NYHA III) and previous cardiac decompensation. On coronary angiography he had three-vessel disease with previous stent implantation in the second left posterolateral branch with complete revascularisation and a good angiographic result. He had severe chronic obstructive pulmonary disease (GOLD IV) with pulmonary hypertension (RV/RA gradient 64 mmHg). Moreover, he suffered from severe peripheral artery disease with asymptomatic high-grade stenosis of the left internal carotid artery and high-grade stenosis of both common iliac arteries and renal insufficiency (GFR 39 ml/min, CKD stage 3B). Previously he had suffered from subacute bleeding in the right basal ganglia without vascular malformation. Vascular risk factors were hypertension, hypercholesterolaemia and smoking. EuroSCORE II was 13% and STS (Society of Thoracic Surgeons) PROM (predicted risk of mortality) score 17%.
The aortic annulus measured by CT was 22 mm in mean diameter. CT was also used to identify the least calcified aortic target facing the inferior caval vein with no interposed structures to determine suitable angiographic projection angles and fluoroscopic landmarks in relation to the lumbar vertebrae. TAVI was performed under general anaesthesia. After puncture of the right femoral vein and the left femoral artery, aortic and caval angiography was performed simultaneously to identify the puncture site (Figure 1A and Moving image 1). An Amplatz GooseNeck Snare (ev3/Covidien, Dublin, Ireland) was placed in the aorta at the expected puncture site as a target to receive the wire used for crossing from the inferior caval vein (Figure 1B). The crossing system consisted of an amputated stiff 0.014 inch guidewire (ASAHI Confianza PRO 9; Abbott Vascular, Santa Clara, CA, USA) inside a 0.035 inch wire converter (PiggyBack; Vascular Solutions, Minneapolis, MN, USA) inside a support catheter (NaviCross™; Terumo, Somerset, NJ, USA) inside a guiding catheter (RDC1; Cordis Corp., Fremont, CA, USA). Crossing was performed during a two-second application of 50 W to the distal guidewire using electrocautery to vaporise surrounding tissue. The guidewire was then captured by the snare and placed in the thoracic aorta. Subsequently, the crossing system was replaced by a rigid 0.035 inch guidewire (Back-Up Meier; Boston Scientific Europe, Ratingen, Germany) and a 14 Fr, 35 cm long Edwards TAVI expandable introducer sheath (eSheath; Edwards Lifesciences, Irvine, CA, USA) was slid from the femoral vein, inferior caval vein, through the caval-aortic tract into the abdominal aorta without dilatation (Figure 1C). TAVI was then performed according to the standard protocol. Following balloon angioplasty with a 20 mm balloon, a 23 mm Edwards SAPIEN 3 valve was implanted under rapid pacing (Figure 1D and Moving image 2). After delivery of the SAPIEN 3 valve through the eSheath and TAVI, digital subtraction angiography of the aorta at the level of the sheath confirmed continued haemostasis. The caval-aortic junction was then closed with a 6 mm AMPLATZER Muscular VSD Occluder (St. Jude Medical, St. Paul, MN, USA) using an 8.5 Fr Agilis™ deflecting sheath (St. Jude Medical) inside the TAVI sheath. Device size was selected to approximate the outer diameter of the sheath (8 mm max diameter during passing of the crimped transcatheter valve) assuming some degree of recoil of the eSheath and the distance between the aorta and the caval vein. The occluder was deployed by exposing the distal disc in the aorta, retracting to apply to the aortic wall and deploying the proximal device near the caval vein. Aortography was performed to ensure no retroperitoneal accumulation of contrast media (Figure 1E). Protamin was applied to reverse heparin anticoagulation. The femoral vein access site was closed using two prepositioned sutures (ProGlide; Abbott Vascular). The postoperative period was uneventful and the patient was discharged four days after the procedure. Before discharge, a CT was performed that showed no extravasation in the retroperitoneal space and only minimal aortic caval shunt (Figure 1F). After this procedure, two further patients were successfully treated with eSheaths during caval-aortic access at the Center for Structural Heart Disease, Division of Cardiology, Henry Ford Health System, Detroit, MI, USA and one patient in the German Heart Center Munich, Germany.
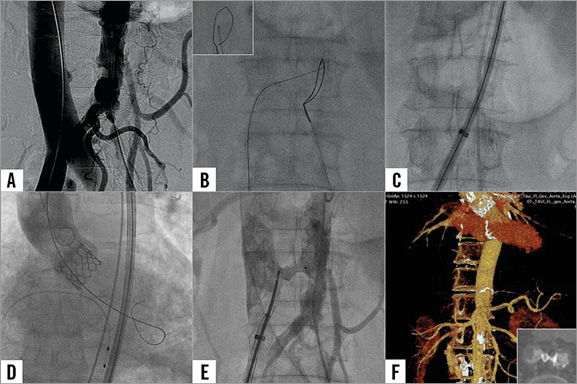
Figure 1. Transcaval access for TAVI implantation. A) Caval and aortic angiography before puncture. B) Caval-aortic crossing with a guidewire, the inset showing fluoroscopy of the lateral view. C) Introduction of the eSheath through the caval-aortic access. D) Aortic angiography after TAVI. E) Closure of the caval-aortic access with an AMPLATZER VSD occluder. F) CT scan before discharge, the inset showing occlusion of the caval-aortic access with the occluder.
Discussion
We report the first TAVI in Europe using caval-aortic access in a patient unsuitable for other access sites. We also report the first implantation of a SAPIEN 3 valve via caval-aortic access and the first use of an expandable eSheath during caval-aortic access.
The rationale for caval-aortic access is that the iliofemoral veins are larger and more compliant than the adjacent arteries. Secondly, the distal inferior caval vein is close to the aorta without interfering structures. Thirdly, large arteriovenous fistulas, such as aortocaval fistulas arising from aneurysm or trauma2 may cause some morbidity but are not immediately life-threatening3. Such fistulas avoid haemorrhage by decompressing high-flow arterial ruptures into the venous system. Similarly, in animal models intentional failure to close the caval-aortic fistula was well tolerated without retroperitoneal bleeding because of the pressure ratio in the retroperitoneal space (pressure aorta >retroperitoneal space >caval vein)4.
Accordingly, in the CT scan shortly after the procedure we observed small amounts of contrast media crossing from the aorta to the caval vein but no contrast media or bleeding into the retroperitoneal space. We used a VSD occluder for closure of the caval-aortic fistula; however, development of dedicated closure devices for this new access route may improve this procedure.
Until this report, all known procedures utilising caval-aortic access had been performed with traditional sheaths “static” in diameter. The concern was that recoil of an expandable sheath after transition of the crimped transcatheter valve through it might result in retroperitoneal bleeding. In our patient and in two further patients afterwards, however, we found no retroperitoneal bleeding after passing the crimped valve through it, showing that transient expansion of the sheath does not enduringly enlarge the caval-aortic tunnel. Thus, the eSheath can be used in patients treated with caval-aortic access, allows the performance of a standardised implantation procedure of the SAPIEN 3 aortic valve utilising the natural recoil properties of the aorta, may allow the use of smaller closure devices, and may lead to more frequent and/or earlier closure of the remaining fistula.
Conclusions
The present case demonstrates the feasibility of TAVI using an eSheath during caval-aortic access in patients unsuitable for other access sites.
Acknowledgements
We thank Marcus Chen, MD, of the National Heart, Lung, and Blood Institute, Bethesda, MD, USA, for analysis and preparation of the transcaval approach.
Conflict of interest statement
M. Kasel and A. Greenbaum are proctor physicians for Edwards Lifesciences. A. Greenbaum is also consultant for Transmural Systems. The other authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. The aortic puncture.
Moving image 2. The TAVI implant.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. The aortic puncture.
Moving image 2. The TAVI implant.

