
CASE SUMMARY
BACKGROUND: A 91-year-old gentleman with a symptomatic severe aortic valve stenosis and chronic kidney disease was referred to our hospital for evaluation.
INVESTIGATION: Valve area: 0.5 cm2 with a mean transaortic gradient of 52 mmHg. EuroSCORE II: 15.24% and STS score: 10%. The Heart Team decided to implant a balloon-expandable Edwards SAPIEN 3 transcatheter valve via a transaortic approach (important peripheral disease).
DIAGNOSIS: During inflation, the Edwards SAPIEN 3 valve expanded asymmetrically and embolised in the ascending aorta where it remained stuck onto the outside lumen of the sheath in a half-deployed conformation.
MANAGEMENT: After different manoeuvres, the valve was successfully uncoupled from the sheath but a complete valve expansion had to be performed, leaving the valve floating in the ascending aorta. Using a transfemoral route, the valve was captured and inflated in the aortic arch.
KEYWORDS: complication, transaortic, transcatheter aortic valve implantation (TAVI)
PRESENTATION OF THE CASE
A 91-year-old gentleman with a symptomatic severe aortic valve stenosis was referred to our hospital for evaluation. His past medical history included previous percutaneous coronary intervention to the left anterior descending coronary artery and chronic kidney disease (GFR 30 mL/min). Transthoracic echocardiography showed a valve area of 0.5 cm2 with a mean transaortic gradient of 52 mmHg and a left ventricular ejection fraction of 45%. A CT scan demonstrated an important peripheral disease including an atherothrombotic disease of the aortic arch. Considering a calculated EuroSCORE II of 15.24% and an STS score of 10% (surgical mortality), the Heart Team decided to implant a 26 mm balloon-expandable SAPIEN 3 transcatheter valve (Edwards Lifesciences, Irvine, CA, USA) via a transaortic approach.
In the cathlab, a 5 cm right mini-thoracotomy at the second intercostal space was performed and a non-calcified entry zone in the ascending aorta was identified. After having performed two concentric reinforced purse-string sutures, a stiff guidewire was placed retrogradely across the stenotic aortic valve followed by the introduction of the Certitude™ sheath (Edwards Lifesciences). During the procedure, difficulties in introducing the valve through the 18 Fr introducer sheath were encountered and crimping was then performed twice, but finally the valve passed through the sheath. Retrospectively, it was observed that the manoeuvre to pass the valve through the sheath shifted the position of the balloon against the stented valve and caused a misalignment of the balloon marker compared to the valve (Figure 1A). Consequently, during valve inflation, the Edwards SAPIEN 3 valve expanded asymmetrically and embolised in the ascending aorta where it remained stuck onto the outside lumen of the sheath in a half-deployed conformation (Figure 1B).
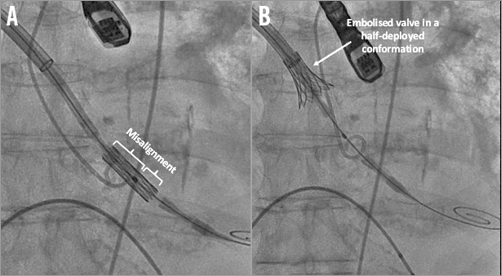
Figure 1. Edwards SAPIEN 3 aortic valve before and after inflation. A) Misalignment of the balloon and the Edwards SAPIEN 3 valve (asymmetric position of the marker). B) Embolised Edwards SAPIEN 3 valve after balloon inflation and stabilisation onto the outside lumen of the sheath.
How would I treat?
THE INVITED EXPERTS’ OPINION

This is an extremely difficult situation requiring ad hoc decision making. Possible options could be:
1. Femoral approach: keep the stiff guidewire within the transcatheter heart valve (THV) to prevent inversion of the THV. After insertion of a second wire through the femoral artery, the deployment balloon is then partially re-inflated within the valve, with the balloon shoulder as a leading edge to reposition the valve atraumatically. This should release the valve from the sheath. The second wire is now passed retrogradely through the balloon-expandable valve and through the native valve into the ventricle. The first wire and sheath are now retrieved through the puncture site of the aorta and the purse-string suture is secured. The SAPIEN valve will now embolise over the wire to the descending aorta. A balloon should be inserted over the wire and inflated inside the SAPIEN valve to fix it in the descending aorta.
A second SAPIEN 3 valve can now be inserted in the customary manner through the puncture site of the aorta.
Potential hazards: the embolised valve must pass through the atherothrombotic aortic arch, insertion of a wire and balloon in the diseased femoral artery. The risk of stroke can be diminished by the insertion of an embolic protection device.
2. Convert to transapical approach, valve deployment in the descending aorta: expose the left ventricular (LV) apex, insert a sheath. Exchange the stiff wire for a long flexible guidewire, which can be captured and externalised through the LV apex using a gooseneck snare. Advance the snare again from the apex on the guidewire into the ascending aorta and capture the cranial part of the valve (inflating the balloon partially while advancing the snare could help this), and pull it back from the sheath. Remove the valve delivery system through the aortic sheath, while the guidewire remains in situ. Now, the valve can be captured with a balloon advanced from the apex on the guidewire.
The guidewire can now be pulled back through the transapical sheath, until it reaches the distal tip of the aortic sheath and enters the aorta. At this point the guidewire should be advanced to the descending aorta.
Following these manoeuvres, the captured valve should be on a partially inflated balloon, completely detached from the aortic sheath. With the snare released, the captured valve can now be advanced to the descending aorta, where it can be safely deployed.
After removing the balloon and replacing the guidewire, one can continue the procedure with the implantation of a second valve transapically.
Drawbacks: second major thoracic incision, manipulation of the apex and the atherothrombotic aorta, risk of stroke. The risk of stroke can be diminished by insertion of an embolic protection device.
3. Convert to transapical approach, then valve-in-valve: convert to transapical, capture and externalise the guidewire through the apex. The guidewire is now crossing the heart from the apex to the aorta. Withdraw the original valve delivery system through the aortic sheath and insert a second SAPIEN valve transapically. After that, capture the embolised valve using a snare and a balloon, pull it back from the transaortic sheath, and deploy it into the aortic position as a valve-in-valve.
Drawbacks: second major incision, manipulation of the apex. Difficult to align the balloon in the valve.
4. Transaortic second valve, then valve-in-valve. First implant a new SAPIEN 3 valve in the proper position using the same sheath, and then re-use that balloon and partially inflate within the dislodged valve. If successful, the assembly of the “partially inflated balloon within the partially expanded dislodged valve” can be advanced into the correctly positioned valve to end with a valve-in-valve.
Drawbacks: re-use of the original (possibly damaged) sheath is needed; it might be difficult to capture the embolised valve properly with a balloon through the transaortic sheath.
5. Should all the above-mentioned strategies fail, the option to retrieve the valve on cardiopulmonary bypass (CPB) during conventional surgery through a full sternotomy, followed by a surgical aortic valve replacement, always exists.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERT’S OPINION

Transcatheter aortic valve embolisation is a rare but often catastrophic complication of transcatheter aortic valve implantation (TAVI). The incidence of embolisation of the SAPIEN valve has declined over time with improved operator experience, use of CT aortic annular measurements to guide sizing, accurate identification of co-planar valve deployment angles, accurate valve prosthesis positioning, and the availability of larger-sized prostheses. However, embolisation still occurs with an incidence of 0.5-0.8%. Most embolisation, as in this case, occurs into the ascending aorta or supra-annular location, but embolisation into the left ventricular outflow tract and left ventricular cavity can also occur. In the published literature, aortic embolisation is managed by placing a second TAVI device and deploying the embolised valve in an aortic location. The embolised valve leaflets remain fully open, essentially functioning as a “covered stent”.
As with any procedural complications, prevention is often better than trying to solve the problem post hoc, so in this case I would start by suggesting that, when there was extreme difficulty in advancing the delivery system into the sheath resulting in movement of the stent-valve frame on the delivery balloon, I would have removed the system and re-prepared the entire delivery system or mounted a new valve and delivery system.
In this situation with a partially deployed flared valve frame stuck on the outside of the introducer sheath, I would try to facilitate completion of the procedure without resorting to cardiac surgery.
It is extremely important that the existing guidewire is maintained in position at all costs. If possible, I would withdraw the current delivery system valvuloplasty balloon back into the sheath and position it with its mid marker at the midpoint of the valve frame. I would then de-sheath the delivery balloon and with rapid pacing re-inflate the embolised valve frame fully. I would then remove the existing delivery system, re-insert the sheath introducer/dilator and re-advance the sheath with its introducer through the embolised valve frame to facilitate unobstructed advancement of a second valve mounted on a new delivery system.
I would then go ahead and perform a standard TAVI implant under rapid RV pacing control with a new 26 mm SAPIEN 3 transcatheter valve delivery system.
Based on the fact that this patient has significant atherothrombotic material in the aortic arch, I would suggest leaving the embolised valve in the ascending aorta and not trying to snare the device and pull it across the aortic arch. I would withdraw the sheath until the embolised valve was fully uncovered. Using preprocedural CT dimensions, I would take an oversized balloon approximating the ascending aortic dimensions and post-dilate the embolised valve frame into position in the ascending aorta.
An alternative strategy would be to deploy a second 26 mm SAPIEN 3 valve in the correct location and then place the patient on femoro-femoral arteriovenous circulatory bypass and open the ascending aorta to remove the embolised valve directly. However, given the patient’s atherosclerotic aortic disease, I feel this would carry a substantial risk of stroke and perioperative morbidity.
Conflict of interest statement
The author has no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
First, digital compressions on the aorta in order to uncouple the valve were attempted, without success. At this moment, due to the haemodynamic instability of the patient in the context of severe aortic regurgitation, the decision to implant a second valve using the same sheath and passing through the half-deployed valve (Figure 2A) was taken. A second valve (26 mm Edwards SAPIEN 3) was successfully implanted without significant residual aortic regurgitation (Figure 2B). Secondly, a surgical aspiration device outside the aorta was used in order to dislocate the stuck valve from the sheath, without success (Figure 3A). Thirdly, after inflating the balloon of the valve (Figure 3B), the valve was successfully uncoupled from the sheath, but at this stage a complete valve expansion had to be performed, leaving the valve floating in the ascending aorta (Figure 4A). This manoeuvre allowed the valve to be captured with another balloon via a transfemoral route and pulled into the aortic arch between the left common carotid artery and the left subclavian artery (Figure 4B). Nevertheless, at this moment, an important aortic dissection was observed. After discussion within the Heart Team, given the patient’s clear instructions not to perform bail-out surgery and the haemodynamic stability, it was decided to stop the procedure. The patient was transferred to the intensive care unit with an antithrombotic therapy and died on postoperative day seven from a major stroke.
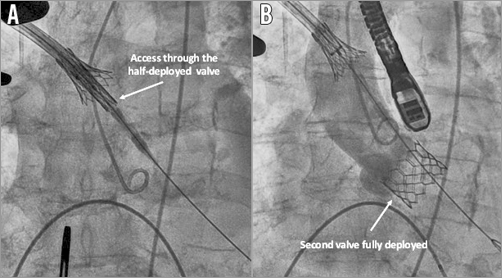
Figure 2. Use of another Edwards SAPIEN 3 aortic valve. A) Access to the aortic valve through the embolised Edwards SAPIEN 3 valve. B) Second Edwards SAPIEN 3 valve correctly positioned.
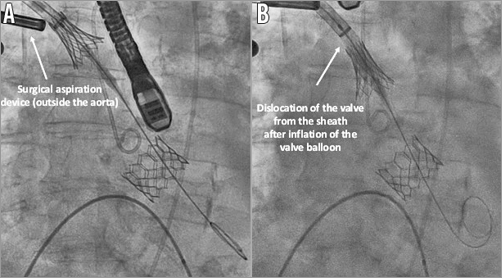
Figure 3. Manoeuvres in order to uncouple the valve from the sheath. A) Use of a surgical aspiration device (outside the aorta) to try to uncouple the Edwards SAPIEN 3 valve from the sheath. B) Second inflation of the embolised Edwards SAPIEN 3 valve.
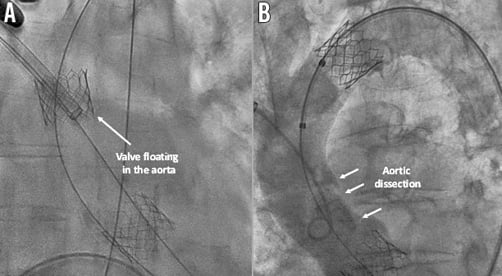
Figure 4. Capture and inflation of the first valve. A) Edwards SAPIEN 3 valve fully expanded but floating in the aorta. B) Aortic dissection and valve in its final position in the aortic arch between the left common carotid artery and the left subclavian artery.
To the best of our knowledge, this is the first report of an aortic valve embolisation via a transaortic approach. In the Polish arm of the ROUTE trial designed to determine the feasibility of transaortic access for transcatheter valve implantation procedures employing the Edwards SAPIEN valves (SAPIEN XT and SAPIEN 3), device success was achieved in 100% of cases1 and device embolisations were not reported. In other smaller series reporting the safety of transaortic procedure, Bapat et al (first published series of the transaortic route with the SAPIEN valve, 17 patients)2 and Clarke et al (SAPIEN valve, 13 patients)3 also did not report this complication. In contrast to transfemoral and transapical procedures where valve embolisation can be managed more easily with favourable outcomes at long-term follow-up4, the transaortic approach is more challenging, and operators should be prepared to face this complication. Indeed, as the guidewire does not cross the arch, an embolised valve requires a rescue transfemoral guiding in order to be moved and inflated in the descending aorta. Furthermore, even if the main reason for valve embolisation is reputed to be a too aortic position4, other causes have been described such as inadequate visualisation of the valve plane. Here, the manoeuvre to pass the valve through the sheath shifted the position of the balloon against the stented valve and created a misalignment of the valve over the balloon.
Conflict of interest statement
The authors have no conflicts of interest to declare.

