CASE SUMMARY
BACKGROUND: Despite the technical advancements of the transcatheter aortic valve implantation (TAVI) procedure, valve embolisation into the left ventricle remains a challenging situation requiring expedited management through the Heart Team.
INVESTIGATION: The advantages and pitfalls of an interventional transfemoral approach, a transapical extraction of the dislocated prosthesis or the conversion to open heart surgery have to be balanced depending on the overall situation and the specific characteristics of the patient.
DIAGNOSIS: A transfemoral approach would be the first choice for most TAVI implanters. We discuss the different options and present an elegant solution solving this challenging situation, leading to a good immediate and long-term outcome.
MANAGEMENT: Attempts at pulling the prosthesis out of the ventricle using a balloon remained unsuccessful. After grasping of the prosthesis with a goose-neck snare, the valve was pulled into the annulus. A second SAPIEN XT prosthesis was implanted and fixed the first prosthesis within the annulus. After post-dilatation, there was a good result without relevant gradient and minimal aortic regurgitation.
KEYWORDS: aortic stenosis, bail-out, percutaneous valve, retrieval
PRESENTATION OF THE CASE
A 73-year-old female patient was admitted in December 2013 with dyspnoea (NYHA Class IV) and marked peripheral oedema. Her medical history included three-vessel coronary disease with coronary bypass surgery in December 2009 (left internal mammary artery to the LAD and vein graft to the obtuse marginal branch), chronic obstructive pulmonary disease, anaemia associated with chronic heart failure and severe retroperitoneal fibrosis. Her cardiovascular risk-factor profile included diabetes mellitus type 2, dyslipidaemia, arterial hypertension and morbid obesity with a body mass index of 43.
Echocardiography showed a high-grade calcified aortic valve stenosis (valve area 0.7 cm2) with severe pulmonary hypertension. Coronary angiography showed stable coronary disease with patent grafts without the need for revascularisation. Due to the high perioperative risk (EuroSCORE II of 20), the previous cardiac surgery and the patient being severely overweight, the Heart Team decision was in favour of a transfemoral TAVI under conscious sedation. The aortic annulus measurements revealed 20 mm on transoesophageal echocardiography and 21 mm on computer tomography, leading to the decision to use an Edwards SAPIEN XT 23 mm balloon-expandable valve (Edwards Lifesciences, Irvine, CA, USA). The intraprocedural balloon sizing based on the predilation supported the choice of the 23 mm prosthesis (Moving image 1). After adequate prepositioning, the valve was implanted under rapid pacing with 200 beats per minute. The stored tension in the catheter system and a breathing manoeuvre of the patient led to the dislocation of the prosthesis into the left ventricle, still centred on the wire (Figure 1, Moving image 2, Moving image 3). Immediate haemodynamic parameters were stable and the Heart Team assessed the treatment options.
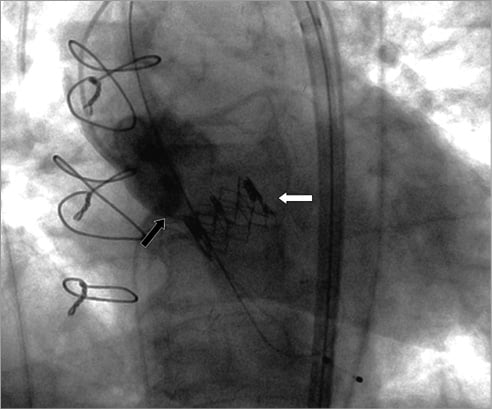
Figure 1. Dislocated valve (white arrow) below the annulus (black arrow) with high mobility.
How would I treat?
THE INVITED EXPERT’S OPINION
The procedural dilemma of an embolised transfemoral Edwards SAPIEN XT valve (Edwards Lifesciences, Irvine, CA, USA) into the ventricle is a difficult one. Some aspects of the procedural set-up may have, at least partially, contributed to this complication. The patient was not under general anaesthesia and therefore there was no transoesophageal imaging. In addition, the technique of stopping ventilation transiently to avoid respiratory movement cannot be performed. Finally, the starting position of the device for deployment was a little low and the inflation was rather quicker than we would perform at my institution.
Prior to any TAVI procedure it is essential for the Heart Team to discuss the “bail-out” options. A pre-procedural decision should have been made as to whether conversion to open surgery should take place.
If this complication occurred at my institution, I think the first consideration would be to put the patient onto femoral bypass if they were haemodynamically unstable. This would of course mean conversion to general anaesthesia. This patient is reported as having been haemodynamically stable, so I would consider the following options in the following order.
The embolised device is free just below the left ventricular outflow tract but remains on the guidewire. This is vital. The first option would be to re-advance the deployment balloon and try to re-capture the device by re-inflating the balloon within the device. Then, without rapid pacing, I would pull the device into the left ventricular outflow tract and add an extra 2 to 3 mls of volume to embed the TAVI valve in the LVOT. If this proved successful, I would then place a second TAVI valve across the aortic valve but overlapping the proximal end of the first device to anchor it in the outflow tract. This would be the solution of choice if it were technically possible.
If the above manoeuvre were not successful, we would convert to the transapical approach. A large sheath would be placed from the apex. The guidewire would be snared and externalised via the sheath. Then, surgical forceps, such as a mosquito clamp, would be used to crush the expanded valve so that it could be withdrawn externally through the sheath along the guidewire1. We would consider the option of introducing a balloon through the TAVI valve before crushing, so that subsequent partial inflation would “guide” the valve out through the apex with less trauma. Following this, a second TAVI valve would be placed in the aortic position under general anaesthesia with transoesophageal echo and using the “breath hold” manoeuvre made possible by the ventilated patient.
If none of the above strategies proved successful, there would be little option other than to convert to open surgery in order to remove the device and, potentially, surgically replace the aortic valve. This would be the option of last resort.
The transfemoral solution would certainly be the optimal one.
Conflict of interest statement
The author has no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION
Prosthesis embolisation immediately after deployment of a transcatheter heart valve is a rare complication and is usually the result of prosthesis/annulus mismatch, unduly high or low implantation, or ejection of the device by an effective ventricular contraction during deployment. Malposition and embolisation are more common with the self-expanding CoreValve® device (Medtronic, Minneapolis, MN, USA)2, and embolisation usually occurs upwards into the aorta. A fully deployed device which has embolised into the aorta can often be snared and repositioned, with or without a partially inflated valvuloplasty balloon, into a more distal stable position. Thereafter, a second valve can be implanted or an alternative approach considered3.
The case reported by Tiroch et al describes a downward embolisation of a balloon-expandable valve into the left ventricle immediately after implantation. This is an extremely rare complication and, fortunately, we have not yet experienced a similar one. Though sizing seems appropriate given the stated dimensions and the intraprocedural balloon sizing, three-dimensional annular measurements (including area and perimeter) are not provided, and the amount and distribution of leaflet and annular calcification are not mentioned. Although balloon-expandable valves usually require less oversizing than self-expanding ones, the presence of calcification is essential for appropriate anchoring, especially if sizing yields a borderline situation.
If such a complication is encountered, maintaining coaxial wire position is important, as it prevents the valve from flipping over to obstruct antegrade flow. As snaring does not seem to be an option here, urgent cardiac surgery should be discussed first. Using conventional sternotomy and cardiopulmonary bypass, the dislocated valve can be removed under direct vision, followed by conventional surgical aortic valve replacement. If sternotomy has to be avoided (previous bypass surgery and patent mammary graft), a further option is to use femoro-femoral cardiopulmonary bypass and remove the valve with a forceps through an apical incision via a small thoracotomy. A larger valve could then be implanted, either transapically or transfemorally. If surgery is not an option, the maintained wire position may facilitate interventional management. In this case, a partially inflated valvuloplasty balloon can be used to drag the prosthesis more proximally in a subannular position under rapid ventricular pacing. This should be followed by the implantation of a second (probably larger) valve, which would help anchoring the dislocated valve and prevent distal migration. In this case, transoesophageal echocardiography would be used for additional guidance and for detection of any possible interaction with the mitral valve apparatus. Ultimately, however, it must be stressed that operator technique and experience play a critical role in the management of such a complication.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
The Heart Team decided against an emergent surgical approach given the excessive operative risk. Pulling of the highly mobile valve was attempted using the SAPIEN balloon system and then using a NuCLEUS 25 mm valvuloplasty balloon (Numed Inc., Hopkinton, NY, USA). The prosthesis centred on the balloon did not pass through the outflow tract and the native valve despite extensive pulling manoeuvres (Figure 2, Moving image 4). Over a second wire placed into the left ventricle, snaring of the vertical valve ridge was successful using an Amplatz GooseNeck™ 25 mm Snare (ev3 Endovascular Inc., Plymouth, MN, USA). The prosthesis was pulled up just above the aortic annulus, but the rotated position induced a moderate to severe aortic regurgitation (Figure 3, Moving image 5). A new SAPIEN XT 23 mm aortic valve (Edwards Lifesciences) was immediately implanted with good, but slightly lower, position due to impingement through the first valve (Moving image 6). This resulted in a good fixation of the previous prosthetic valve and an aortic regurgitation grade II. A final post-dilation was performed with the NuCLEUS 25 mm balloon covering both valves leading to a minimal residual regurgitation (Figure 4, Moving image 7, Moving image 8). The maximum gradient was 5 mmHg. Despite the lower position of the prosthesis, only a mild mitral regurgitation was present. The bypass grafts ensured good coronary perfusion. The patient was discharged eight days after the intervention without further events. She showed a marked clinical improvement with dyspnoea NYHA Class I, preserved at six-month clinical follow-up.
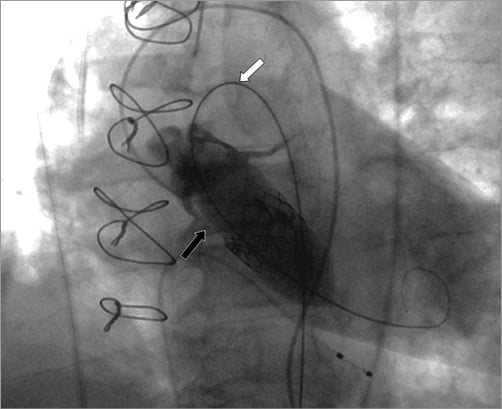
Figure 2. Minimal protrusion of the balloon into the aorta just above the annulus (black arrow) despite extensive tension on the balloon with wire on the inner curvature of the aortic arch (white arrow).
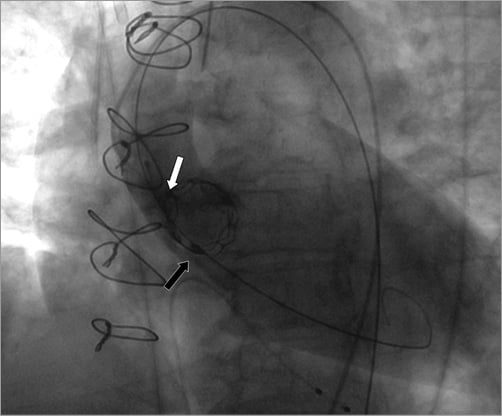
Figure 3. Attachment of the snare at the vertical valve ridge (white arrow) and pulling of the valve just above the annulus (black arrow).
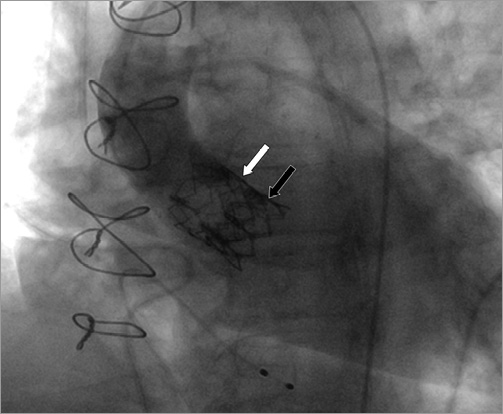
Figure 4. Tilted first prosthesis (white arrow) after snaring and fixation with second prosthesis within the annulus (black arrow), leading to a good final result.
Dislocation of the valve into the left ventricle is a rare but feared complication of the widely used balloon-expandable SAPIEN prosthesis. A large meta-analysis of almost 10,000 TAVI patients identified valve dislocation as the most common cause for emergent cardiac surgery, resulting in a high mortality in this extreme risk situation4,5. Our literature search regarding the approach for a dislocated SAPIEN prosthesis into the ventricle yielded only a few case reports, with all patients undergoing emergent cardiac surgery, most often with transapical grasping attempts6,7. Patients survived in most case reports, but underreporting of fatal outcomes during such extreme risk procedures is likely.
Interventional approaches to extract the dislocated valve from the ventricle are obviously associated with a high risk, but the overall risk of emergent surgery might be even higher. To our knowledge, we present the first case of a dislocated SAPIEN prosthesis into the left ventricle, addressing the advantages and pitfalls of balloon pulling and subsequent successful snaring.
Keeping the valve centred on the wire is essential, and pulling the prosthesis with the SAPIEN balloon system is an obvious choice. However, based on our case, the probability of a successful repositioning of the valve within the annulus is quite low. The valve on the balloon will either not pass through the annulus given the larger balloon diameter, or might injure the outflow tract or aortic annulus. With smaller balloons, the valve will probably shear off within the left ventricular outflow tract. If the valve remains stable within the upper left ventricular outflow tract, fixation of the valve with a second prosthesis can be attempted, but a high mobility of the dislocated valve excludes this option. Snaring of the prosthesis will lead to a slight deformation of the valve with a higher likelihood of passage through the left ventricular outflow tract and the annulus. The required pulling forces are lower, allowing a rather controlled pulling and positioning either within the annulus, as in our case, or into the descending aorta with alignment and attachment with a subsequent post-dilatation or stenting. Grasping of the valve with a goose-neck snare will succeed most likely at the cranial part of the vertical ridges of the SAPIEN XT valve, and at the attachment sites of the pericardium for the SAPIEN 3 valve.
A dislocated SAPIEN prosthesis can be successfully pulled out of the ventricle using a goose-neck snare. Pulling manoeuvres with a balloon failed due to the larger diameter compared to the annular structures.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Predilation with concomitant sizing, no passage of contrast dye next to the 20 mm balloon.
Moving image 2. Implantation of the Edwards SAPIEN XT 23 mm prosthesis with breathing manoeuvre just before expansion.
Moving image 3. Dislocated valve below the annulus with high mobility.
Moving image 4. Minimal protrusion of the balloon into the aorta just above the annulus, despite extensive tension on the balloon with wire on the inner curvature of the aortic arch.
Moving image 5. Rotated position of the valve just above the annulus after attachment of the snare at the vertical valve ridge, snare already detached.
Moving image 6. Implantation of the second Edwards SAPIEN XT 23 mm prosthesis within the tilted first prosthesis.
Moving image 7. Post-dilation of both prostheses due to moderate aortic regurgitation after second implantation.
Moving image 8. Good final result without relevant gradient or aortic regurgitation.

