Case summary
Background: A 70-year-old man diagnosed with hypertension, severe chronic obstructive lung disease and critical aortic stenosis symptomatic by NYHA functional class III heart failure and frequent hospitalisations for volume overload was referred for percutaneous aortic valve implantation.
Investigation: Cardiac catheterisation revealed a severe aortic stenosis with a peak to peak gradient of 95 mmHg and a mean gradient of 73 mmHg. Transesophageal echocardiography demonstrated an aortic valve area of 0.7 cm2.
Diagnosis: Severe symptomatic aortic stenosis by echocardiography and cardiac catheterisation.
Treatment: Transcatheter aortic valve replacement with an 29 mm CoreValve prosthesis.
Keywords: aortic valve stenosis, TAVI
How should I treat?
Presentation of the case
Introduction
Over the last few years transcatheter heart valve implantation has become a viable alternative for the growing number of older, high-risk patients who are not candidates for conventional surgery1,2. One major limitation of the current available prosthesis is that re-positioning or retrieval is very difficult or impossible once deployed.
Case report
A 70-year-old man diagnosed with hypertension, severe chronic obstructive lung disease and critical aortic stenosis symptomatic by NYHA functional class III heart failure and frequent hospitalisations for volume overload was referred for percutaneous aortic valve implantation. Cardiac catheterisation revealed a severe aortic stenosis with a peak to peak gradient of 95 mmHg and a mean gradient of 73 mmHg. Transesophageal echocardiography demonstrated an aortic valve area of 0.7 cm2. Measured dimensions included an aortic annulus of 25 mm, a sinus of valsalva of 30 mm, a sinotubular junction of 27 mm and an ascending aorta of 29 mm. The initial blood pressure was 110/70 mm Hg.
The aortic valve was predilated with a 25 mm balloon facilitated by rapid ventricular pacing. Thereafter, a 29 mm CoreValve (Medtronic, Minneapolis, MN, USA) prosthesis was advanced to the native valve location, positioned at the level of aortic valvular calcification and deployed under fluoroscopic guidance. Proper valve placement was complicated due to a relatively straight axis direction of the aortic valve, and by angiography, it appeared that the valve was implanted slightly too low. Aortography and echocardiography showed the presence of a moderate paravalvular aortic regurgitation with an aortic diastolic pressure of 40 mmHg. The aortic insufficiency was well tolerated without progression of dyspnea or haemodynamic instability and the procedure was terminated with plans for close clinical follow-up.
Over the ensuing few days the patient suffered from dyspnea and orthopnea. Chest x-ray showed pulmonary congestion and a transthoracic echocardiogram demonstrated persistent moderate aortic regurgitation. Orthopnea and pulmonary congestion improved in response to intensive diuretic therapy but another transthoracic echocardiogram performed after two weeks showed increased aortic regurgitation maybe due to further migration of the prosthesis. Repeated measurements of blood pressure showed diastolic values ≤40 mmHg. Eighteen days after the initial procedure, repeat catheterisation and transesophageal echocardiography was undertaken to re-evaluate the position of the valve. Both modalities confirmed severe aortic regurgitation and showed that the valve was sitting far too low i.e., depth of implantation from the right or non-coronary and left coronary cusp to the ventricular edge of the valve frame >12 mm, especially in the region of the left coronary cusp (Figures 1 and 2).
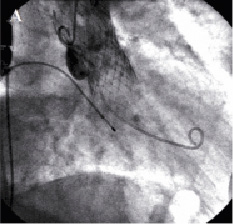
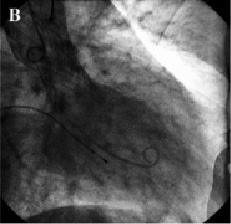
Figure 1. Fluoroscopic appearance of the aortic prosthesis before repositioning. Aortography showed that the valve was implanted too low (A). Severe regurgitation (B).
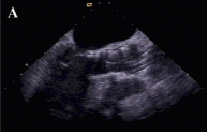
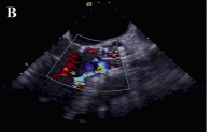
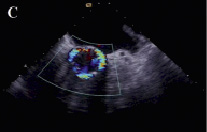
Figure 2. Transesophageal echocardiographic appearance of the aortic prosthesis before repositioning. The valve is sitting far too low (A). Severe paravalvular aortic regurgitation (B,C).
How could I treat?
The Invited Experts’ opinion
The problem of aortic regurgitation (AR) described by Majunke et al may occur using either one of the presently available devices for transcatheter aortic valve implantation (TAVI). Up to one third of patients undergoing this procedure end up with a higher grade of AR post- compared to pre-intervention, one third stay the same and one third improve1.
However, procedure-related impairment of AR usually means a shift from grade 0 to 1+ or 1+ to 2+ and haemodynamically and clinically significant severe AR - almost exclusively paravalvular - at the very end of the procedure occurs in less than 2% of patients1. Potential mechanisms for paravalvular regurgitation are 1) device/annulus mismatch, 2) device malpositioning (too low – too high) and 3) native valve degenerative patterns preventing optimal device expansion (e.g., specific calcification patterns) with resulting gap formations. Optimal pre-procedural evaluation aims to eliminate mechanism, optimal implantation eliminates mechanism and both careful screening and optimal implantation techniques help to deal with mechanism.
The beauty of the self-expanding Medtronic CoreValve Revalving prosthesis used in the present case is the availability of various corrective measures, which can be utilised once either malpositioning or severe AR occurs despite correct positioning.
The bundle of interventional options includes post-dilation using oversized balloons, pull-up manoeuvres using an inflated balloon under rapid ventricular stimulation or preferred, pull-up manoeuvres using a snare attached to one of the two frame loops of the device (rarely one has to use two snares to pull on both loops simultaneously, sometimes one has to modify the traction angle using different access sites – femoral versus brachial). If everything fails, a second prosthesis (Medtronic CoreValve or Edwards) may be implanted either within the first one at a slightly different implantation depth, or within the native valve after pulling back the first prosthesis into the aorta.
Conversion to conventional surgery is theoretically an option but understandably not the favoured one as the patients currently undergoing TAVI are considered high-risk surgical candidates.
In the present case, I would have attempted to optimise the result already during the index procedure immediately after device implantation at which time both “moderate” AR and a decrease of the diastolic pressure from 70 mmHg to 40 mmHg were observed. If the implantation level is not too deep, I would have used the simplest of the listed techniques first: post-dilation with a large balloon (e.g., 28 mm). In case of no effect or clear evidence of a deep implantation, which was probably the case here, I would have attempted to perform a snare manoeuvre, although the threshold to do this in a clinically stable patient with normal ejection fraction with “only” moderate AR is certainly higher than in unstable patients, patients with moderate AR associated with reduced EF and dilated ventricles or severe AR. If I had waited and accepted the AR as reported in this case, I would have brought the patient back to the catheterisation laboratory upon further clinical deterioration as described here and would have attempted to improve the position of the prosthesis by performing a snare manoeuvre as described above. In case of failure, I would have proceeded with the implantation of a second prosthesis within the first frame aiming at a slightly higher position.
Reference
How could I treat?
The Invited Experts’ opinion
The transcatheter aortic valve implantation (TAVI) procedure with the self-expanding CoreValve (Medtronic, Inc., Minneapolis, MN, USA) can be complicated by an “implant failure”1, defined as a suboptimal implant with impairment of the valve performance, despite a good function of the device.
The accurate measurement of the aortic valve apparatus (annulus, sinus of Valsalva width and height) are of critical importance for choosing the correct prosthesis size.
When a paravalvular leak is observed after the CoreValve implantation the severity and the mechanism of the paraprosthetic leak must be addressed.
The severity of the regurgitation must be assessed by haemodynamic measurement, angiographic and echocardiographic evaluation.
The aortic regurgitation is likely to be severe and must be corrected when the diastolic aortic pressure is below 40 mmHg and the LV end-diastolic pressure is higher than baseline. Aortography should be performed with adequate volume and rate of contrast injection in two orthogonal views in order to:
– Quantify the severity of regurgitation
– Identify the location and mechanism of the leak
– Assess prosthesis expansion
– Assess the position of the valve respect to the annulus
The trans-thoracic echocardiography (TTE) is a useful diagnostic tool for identifying the location and extension of the leak and the severity of regurgitation.
The mechanisms of severe paravalvular leak can be:
– Prosthetic valve under-expansion caused by inadequate pre-implant aortic balloon valvuloplasty.
– Low valve deployment, in this case the inlet part of the skirt does not seal the annulus and the blood returns into the LV2;
– High valve deployment, above the aortic cusps, with potential impairment of coronary blood flow.
– Undersized prosthesis diameter respect to the real aortic valve annulus diameter.
If there is a clear under expansion of the valve, or malapposition of one side of the valve, it should post-dilate the inner part of the valve; careful right ventricular pacing has to be performed during inflation in order to reduce the left ventricular systolic pressure and obtain a stable balloon position without any movement which can potentially damage the valve leaflets.
In case of an undersized valve, it is possible to retrieve and place the valve in a safe position in the ascending or descending aorta using a snare and to implant a larger prosthesis3.
If the valve is too high, the valve must be pulled in the ascending aorta, because the risk of coronary flow obstruction. Then place a second valve in a “in-series” manner3.
If the valve is too low, firstly one should try to pull back the valve of few millimetres using a snare with the “snaring” technique1: snare one of the two hooks and start pulling when the beating heart is filled, then stop, maintain the position, wait for few minutes and then start again. This can take time but can be very effective. Monitoring the aortic diastolic pressure is very useful, when it abruptly raises above 50-60 mmHg, it means that the valve is in a satisfying position. When this manoeuvre is ineffective, the “valve-in-valve” technique is the second option2, placing a second valve within the first one, in a standard fashion2.
In the case reported the aortic valve annulus measured is 25 mm, and a large CoreValve 29 mm has been implanted in a lower position with regurgitation from all around the circumference as showed by the short axis view at TTE.
The first manoeuvre that should be adopted is the “snaring” technique. If the valve does not move the hypothesis to put a second valve inside the first one should be considered. If despite the optimal repositioning of the valve to a higher position, the haemodynamics do not improve, the prosthesis can be post-dilated with a 28-mm balloon.
How did I treat?
Actual treatment and management of the case
In view of the above, it was decided to reposition the valve. A 6 Fr sheath was placed in the right brachial artery. A snare was advanced to the aortic valve and one of the loading hooks in an antero-lateral position of the upper part of the prosthesis was successfully snared. Under constant angiographic and transesophageal echocardiographic guidance, mild tension was applied over a period of one hour to bring the prosthesis into a better position. As the valve started moving, we immediately released the tension and the valve stopped 6 mm more cranial as measured on transesophageal echo. After repositioning the prosthesis, the aortic diastolic pressure increased to 50 mmHg and only mild aortic regurgitation was observed on aortography and transesophageal echo (Figures 3 and 4).
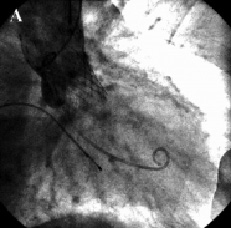
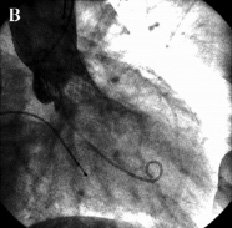
Figure 3. Fluoroscopic appearance of the aortic prosthesis after repositioning. Aortography showed a better position of the prosthesis (A) which resulted in a decrease of aortic regurgitation to mild (B).
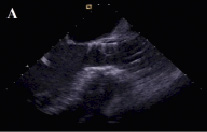
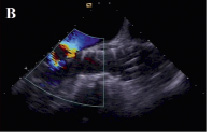
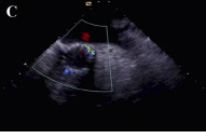
Figure 4. Transesophageal echocardiographic appearance of the aortic prosthesis after repositioning. The position of the valve is much better now (A) paravalvular aortic regurgitation has decreased to mild (B,C).
Transthoracic echo the next day and one week after the second procedure confirmed a stable well-seated prosthesis with only mild paravalvular aortic regurgitation. The remainder of the patient’s hospital course was unremarkable and blood pressure measurements showed continuous diastolic values ≥ 60 mmHg. The patient was discharged in NYHA functional class I and is still doing well seven months later.
Discussion
Details of the CoreValve Revalving System and the technical aspects of the procedure have been described in detail previously3. One potential complication is malpositioning of the prosthesis. There is no exact definition of the ideal depth of implantation but it is recommended to position the ventricular end of the prosthesis ~8 mm below the annulus of the aortic valve to achieve the optimal location of the device and to minimise the risk of complete heart block6. If positioned too high, (i.e., the skirt of the prosthesis is placed above the aortic annulus) paravalvular leak may occur or the valve may dislodge into the aorta. If it is positioned too low (i.e., the prosthesis is implanted so low that the skirt of the prosthesis is positioned below the aortic annulus) it may result in severe aortic regurgitation with haemodynamic compromise due to paravalvular leak. Based on the assumption that repositioning of the CoreValve prosthesis after inadequate positioning is impossible, additional interventions by either second device implantation (valve-in-valve) or conversion to surgery have become options to combat suboptimal device placement. Grube et al3 reported that conversion to surgery or implantation of a second valve was necessary in eight patients (9%) because of suboptimal placement of a first prosthesis. In six patients (7%), misplacement of the valve led to urgent conversion to operative valve replacement, and in two patients (2%), a second CoreValve prosthesis was implanted due to remaining aortic regurgitation. In the CoreValve Registry of 646 patients1, 2.6% of the patients required a second valve; however, no distinction was made between a “valve in valve” or sequential valve implantation. Piazza et al4 reported that a valve-in-valve procedure was performed in five out of 59 patients (8.5%). In two patients the first valve was implanted too high and migrated into the aortic root, while in three patients, the valve was implanted too low. Second valve implantation was successful in all patients, and aortic regurgitation mitigated in all. Ruiz et al5 reported on the long-term outcome of the first valve-in-valve procedure three years after implantation of two CoreValve prosthesis, and demonstrated that the valves continue to function well. Although implantation of a second valve due to misplacement of the first implant is feasible and potentially efficacious, it remains technically challenging and long-term durability, efficacy and safety is still unclear. Vavouranakis et al7 described the “snare technique”. A too low implanted CoreValve prosthesis was snared and successfully repositioned using a constant withdrawal force. This technique was successfully used during the initial procedure immediately after the valve prosthesis was implanted. Nothing is known about the mid- and long-term function of the valve after repositioning.
Here we present the use of a similar technique for treatment of an inferior malpositioning of a CoreValve prosthesis eighteen days after valve implantation. A transcatheter snare technique resulted in successful repositioning of the prosthesis. Most importantly, repositioning resulted in haemodynamic and clinical improvement, hospital discharge and sustained benefit.
In this case, specific attention was paid to the exact moment that the valve began to migrate in order to avoid dislodging the entire device into the ascending aorta. Furthermore, the reproducibility of this technique is uncertain, repositioning was successful in this case, it is unknown what happens to the native valve after being compressed for a period of time. Therefore this technique should be performed only by experienced interventionalists and in the event of dislodgement, everything should be prepared to be able to implant a second valve immediately or proceed with surgical aortic valve replacement as necessary.

