Abstract
Aims: To evaluate the two-step inflation technique aimed at achieving optimal valve implantation depth (defined as 40% of prosthesis height extending below the lower sinus border on angiography) during balloon-expandable transcatheter aortic valve implantation (TAVI).
Methods and results: Between September 2010 and March 2013, 103 patients (67 females, mean age 80.9±5.6 years) were treated with the Edwards SAPIEN XT prosthesis using the two-step inflation technique. Implantation depth was measured on angiography. A historical control group (treated with Edwards SAPIEN) was used for comparison (n=20). Deviation from the defined optimum implantation depth (expressed as a percentage of stent frame height) was significantly less in the study group versus controls (7.0 [3.4-14.1]% vs. 13.9 [5.4-18.9]%; p=0.048). Valve placement was graded “as intended”/“within range”/“out of range” (defined as ≤10%, >10% but ≤20% and >20% deviation, respectively) in 66%/22%/12% of the study group and 35%/40%/25% of historical controls (p=0.02). Corrections in valve position were made in 20 procedures (20%), resulting in placement as intended in 16 cases (80%), with highest efficacy in the transapical and direct aortic approaches.
Conclusions: The two-step inflation technique improves valve placement towards optimal implantation depth and may thereby prevent adverse events due to malpositioning.
Introduction
Correct valve prosthesis positioning is of paramount importance in transcatheter aortic valve implantation (TAVI). Positioning is of particular concern when implanting balloon-expandable valves, since this valve type cannot be retrieved. Incorrect valve placement may lead to serious adverse events, i.e., high-grade aortic valve regurgitation (AR), coronary artery obstruction and valve dislocation1-5. In some patients, valve positioning can be troublesome due to cardiac motion and flow-mediated movement of the deployment balloon, as both are still present to a varying extent during rapid ventricular pacing (RVP). The upper movement phenomenon also adds to the challenge of correct valve positioning6.
In order to optimise valve placement, we introduced a “two-step inflation technique”: a staged inflation of the balloon crimped valve, interrupted by angiographic verification of valve position, all during continuous RVP, to allow for small “fine-tuning” corrections before definite deployment. A comparable technique has previously been described by Pasic et al, exclusively in transapical aortic valve implantation7. The transapical approach may be considered more apt for final valve position adjustments, since this approach provides more direct control. The aim of this study was to evaluate the two-step inflation technique in patients undergoing TAVI using various approaches (transfemoral, transapical and direct aortic), with respect to: 1) feasibility of peri-deployment adjustments in valve position, 2) efficacy in achieving optimal valve implantation depth, and 3) technique safety.
Methods
For a comprehensive description of study methodology, please see the online supplementary methods section (Online Appendix 1).
STUDY DESIGN AND PATIENT SELECTION
This is a retrospective single-centre study. All patients who underwent TAVI at the University Medical Centre Utrecht using the two-step inflation technique were identified retrospectively. For comparison, a historical control group of 20 patients was selected. Preconditions for patient inclusion in this study were: availability of intraprocedural angiographic images, presence of post-deployment aortographic injection in the same projection used for valve implantation, and no overprojection of procedural material obscuring the lower aortic sinus borders.
All periprocedural data were retrospectively collected and registered in a database. Follow-up was retrospectively obtained using documentation of standard of care outpatient visits approximately six months following TAVI. All patients gave informed consent for the procedure and, due to the retrospective nature of the study design, ethics committee approval was waived.
IMPLANTATION PROCEDURE AND TECHNIQUE
The balloon-expandable SAPIEN and SAPIEN XT valves (both Edwards Lifesciences Corp., Irvine, CA, USA) were used in this cohort. Both valves consist of a tri-leaflet bovine pericardial valve sewn into a stainless steel (SAPIEN) or cobalt-chromium (SAPIEN XT) frame, partially covered with a synthetic fabric sealing cuff.
TAVI was performed by transfemoral, direct aortic or transapical approach, depending on the presence of suitable vascular access. In transfemoral procedures conscious sedation was preferably used; otherwise (in the initial femoral, direct aortic or transapical approach) general anaesthesia was instituted. The majority of transfemoral procedures were assisted by intracardiac echocardiography (ICE), for monitoring of potential complications and assessment of prosthesis function. During the initial femoral procedures and all the non-femoral procedures imaging assistance was accounted for by TEE.
Common access techniques were used for TAVI. After establishing access, the procedure continued with identification of the angiographic projection perpendicular to the native aortic annulus. Predilatation was then routinely performed to prepare the device landing zone. The next step was the advancement of the prosthesis towards the aortic annulus for initial placement, verifying the position with angiography (Figure 1A). In transfemoral procedures, initial positioning was done slightly low on purpose, as pulling the system was regarded as safer than pushing it. When valve position was satisfactory, RVP (180-200 bpm) was started, followed by partial inflation of the balloon crimped valve (±1/3 of the full deployment contrast volume) instead of instant complete inflation, the “first step”. Inflation was then interrupted for angiographic reassessment of valve position, which importantly could still be adjusted at that time if deemed necessary for optimisation of implantation depth (Figure 1B and Figure 1C). Finally, when the supposedly desired placement was achieved, the prosthesis was fully deployed by complete inflation of the balloon, the “second step”. All these steps occurred during continuous uninterrupted RVP which would only be stopped after final valve deployment. Following final deployment, prosthesis position and function as well as patency of the coronary ostia were assessed by angiography (Figure 1D). Valvular function was thereafter examined by ICE or TEE in both the short and long-axis views. Additionally, post-dilatation could be performed to address significant prosthetic regurgitation, if incomplete expansion of the prosthesis was suspected. In case of an unsatisfactory result, the decision to implant a second valve was left to the operator’s discretion.
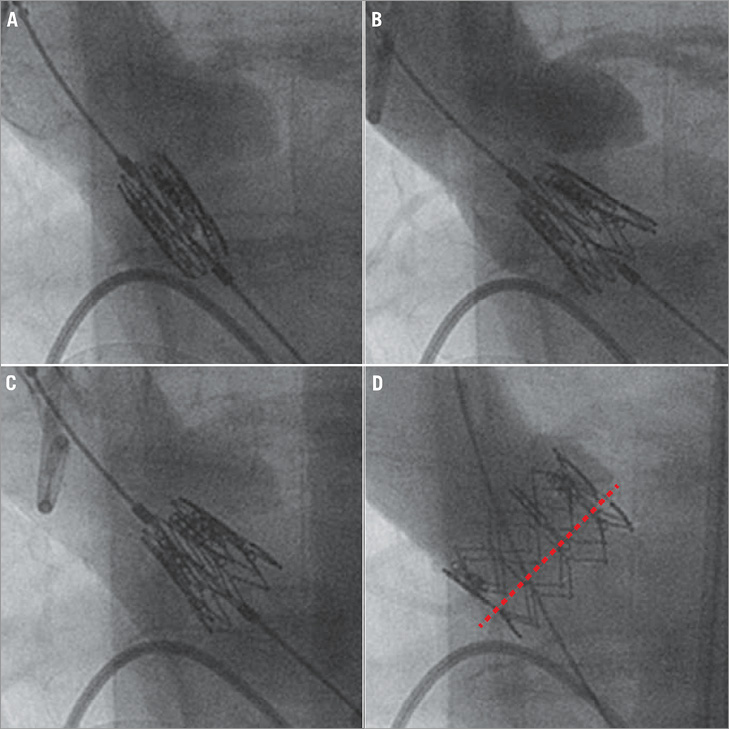
Figure 1. The two-step inflation technique in transfemoral TAVI. A) Aortic angiography is performed for positioning the prosthesis in the aortic annulus. B) After first step inflation, angiography is repeated, revealing a too low position of the prosthesis. C) Position is then adjusted by gently pulling the prosthesis upwards. D) After final deployment a correct position of the valve with respect to the aortic annulus plane (red dotted line) can be appreciated.
ANGIOGRAPHIC MEASUREMENTS
Angiographic measurements were performed offline using dedicated analysis software (CAAS; Pie Medical Imaging, Maastricht, The Netherlands). Valve implantation depth was measured on angiography in the same projection used for implantation, according to the routine described in Figure 2. To compensate for projection errors, the depth of the prosthesis was expressed as a percentage of total prosthesis stent frame height, as previously proposed8.
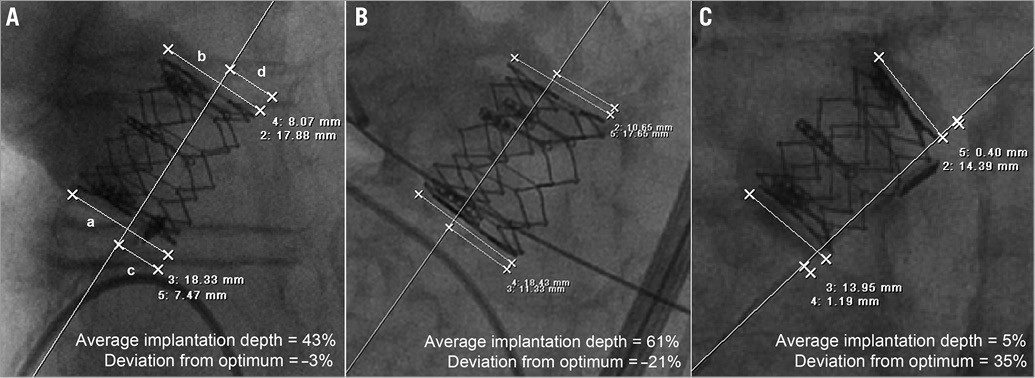
Figure 2. Angiographic measurement of valve implantation depth. A) Valve height was measured at both left (a) and right (b) stent frame edges, as was the portion of stent frame extending below the lower sinus (c and d). Average implantation depth was calculated in percentages of the total stent frame height using the formula [(c/a)+(d/b)]/2*100. Examples are provided for correct (A), too low (B) and too high (C) valve placement.
DEFINITIONS
The optimal implantation depth for the SAPIEN valves used in this study is expressed as a percentage of total stent frame height extending below the lower sinus border on angiography, to enable the use of one single value across the entire range of valve sizes. For evaluation purposes, optimal depth was defined in line with the valve position aimed for during our SAPIEN implantations, namely at 40%. This optimal depth is based on several considerations concerning valve design (height of the fabric cuff) and aortic root anatomy (the proximity of coronary artery ostia and the notion that the “real” aortic annulus is located a little lower than appreciated on angiography). Importantly, the optimal implantation depth definition originates from institutional consensus and lacks scientific evidence for favourable effect on clinical outcome. Final valve position was arbitrarily classified according to the degree of average implantation depth deviation from the specified optimum, as follows: ≤10% deviation was labelled “as intended”, >10% but ≤20% “within range”, and >20% “out of range” (Figure 3).
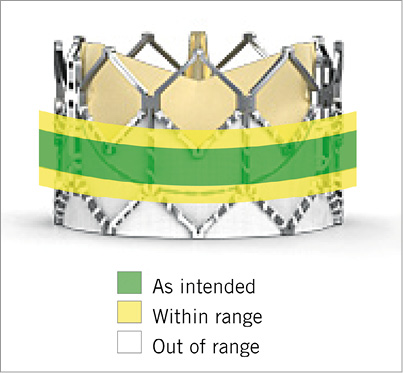
Figure 3. Valve placement classes depicted in a 26 mm Edwards SAPIEN XT valve.
To evaluate the efficacy of peri-deployment valve position adjustments, the study group was categorised into “no-adjustment” and “adjustment” subgroups, depending on whether the use of stepwise inflation led to corrections in valve position. For evaluation of technique safety a composite safety endpoint was created, including mortality, coronary artery obstruction, stroke, pacemaker implantation, myocardial infarction, periprocedural ventricular fibrillation and valve dislocation. Acute procedural success was defined as the successful implantation of a single valve with proper functioning and the patient leaving the operating room alive. Device success was defined according to the VARC-2 criteria.
STATISTICAL ANALYSIS
Data were analysed using IBM SPSS Statistics software version 20 (IBM Corp., Armonk, NY, USA). Continuous variables are presented as means±SD or medians and interquartile range, as considered appropriate, and categorical variables as counts and percentages. For comparison of continuous variables between groups the Student’s t-test or its non-parametric equivalents were used, depending on data distribution. Categorical variables were compared using chi-square or Fisher’s exact test. To account for differences in the study and control group a propensity analysis was performed by means of binary logistic regression, incorporating group membership and the propensity score (propensity to be treated using two-step inflation estimated by non-parsimonious binary logistic regression). The result is reported as an odds ratio (OR) with confidence interval. Two-tailed p-values <0.05 were considered statistically significant.
Results
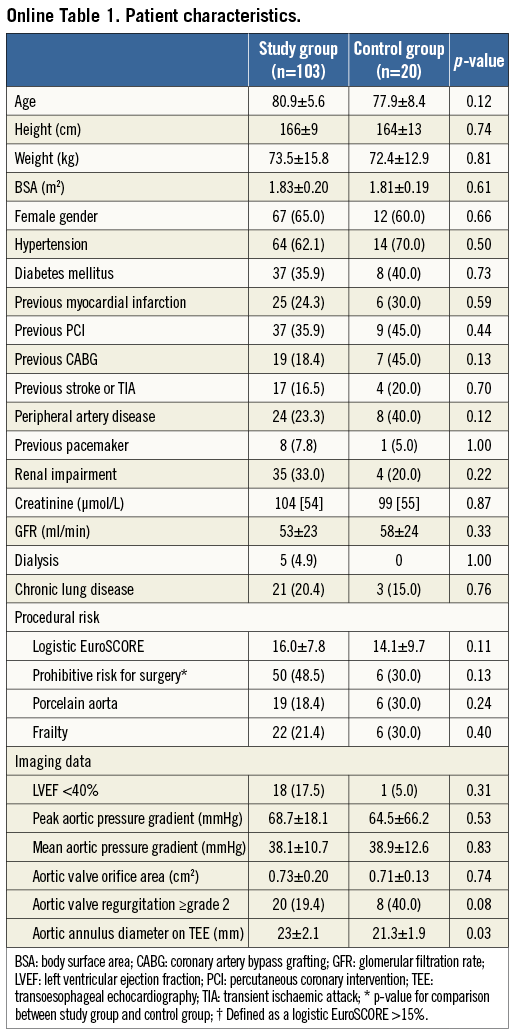
Between the introduction of the two-step inflation technique in September 2010 and March 2013, out of a total of 172 patients treated with TAVI, 54 received a self-expanding valve and 118 a balloon-expandable valve. Amongst the group treated with a balloon-expandable valve, 103 patients fulfilled the inclusion criteria (mean age 80.9±5.6 years, 65.0% female). Fifteen patients were excluded from the analysis: seven (5.9%) patients because procedural material obscured the lower sinus border, four (3.4%) due to post-deployment aortographic injection in a projection different from the implantation view, two (1.7%) because of the absence of angiographic images and two (1.7%) as two-step inflation was omitted. As the technique was introduced after the start of the TAVI programme in our institution, a conventional deployment technique was used in the first 36 patients undergoing balloon-expandable TAVI, of whom the last 20 were selected as a historical control group (mean age 77.9±8.4 years, 60.0% female). Baseline patient characteristics are presented online (Online Table 1). The intraobserver variability for angiographic assessment of average implantation depth was good (0.3±3.7%, paired sample correlation r=0.97; p<0.01), as assessed in 20 randomly selected patients.
PROCEDURAL RESULTS
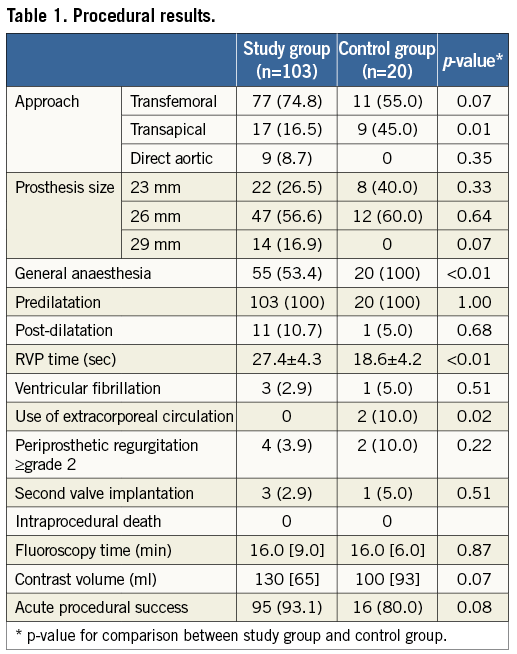
An overview of procedural results is given in Table 1. The majority of implantations in both the study group and the control group were performed by transfemoral approach (74.8 and 55.0%, respectively), followed by the transapical approach (16.5 and 45.0%). The direct aortic approach was only applied in the study group (8.7%). All procedures in the control group were performed under general anaesthesia. RVP time was significantly longer in the study group (27.4±4.3 vs. 18.6±4.2 sec; p<0.01). Valve position was adjusted after first step inflation in 20 (19.4%) patients, who constituted the “adjustment” subgroup. Adjustments were performed in 66.7% of direct aortic, 15.6% of transfemoral and 11.8% of transapical procedures, predominantly towards a more proximal position (60.0% of cases). The extent of valve position adjustment amounted to 16.2±5.7 of stent frame height, corresponding to 2.7±0.9 mm.
There was no intraprocedural mortality. Second valve implantation was performed in three cases of the study group (2.9%) versus one case in the control group (5.0%), exclusively concerning valve-in-valve implantations in the same setting. In the study group, second valve implantation was due to prosthetic valve leaflet immobility (frozen leaflet) causing severe transvalvular AR that was resistant to manipulations with a pigtail catheter (n=2)9 or significant central AR following post-dilatation (n=1). In the control group, one second valve was deployed to treat significant paravalvular AR due to too low implantation of the first valve.
Post-dilatation was applied in 10.7% of patients in the study group (n=11) and 5.0% of historical controls (n=1). The incidence of periprocedural ventricular fibrillation was 2.9% in the study group (n=3) versus 5.0% in the control group (n=1). All cases occurred directly following valve deployment and could be easily resolved by DC shock leaving no sequelae. Acute procedural success was comparable in the study and control groups (93.1% and 80.0%, respectively).
VALVE PLACEMENT
Median deviation of the average implantation depth from the defined optimum was 7.0% [3.4-14.1] for the study group and 13.9% [5.4-18.9] for the control group (p<0.01) (Table 2). Values for individual patients are presented in Figure 4. Valve placement class for each group is presented in Figure 5. The difference in distribution of valve placement class between the study and control groups was significant (p=0.02) with a pronounced higher rate of as intended valve placement in the study group (66.0 vs. 35.0%; p<0.01). After adjusting for differences in baseline characteristics by means of propensity score analysis, two-step inflation was still positively associated with as intended valve placement (OR 5.3; CI: 1.4-19.2; p=0.01). Despite the possibility of peri-deployment corrections, the study group still counted 13 cases (15.7%) of out of range final valve positioning. In both study and control groups, valve placements that did not match implantation depth as intended predominantly comprised too high deployments (72.2% and 92.3%; p=0.24, respectively)
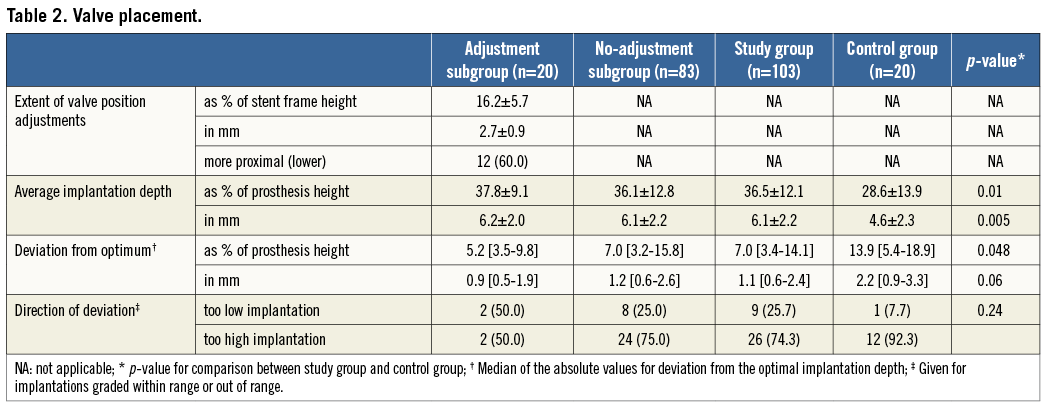
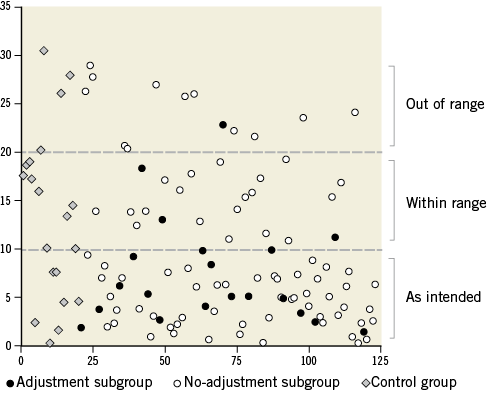
Figure 4. Deviation of valve implantation depth from the optimum. Given for consecutive patients in percentages of stent frame height, presented for all groups in absolute values. Valve placement classes are separated by the dotted lines.
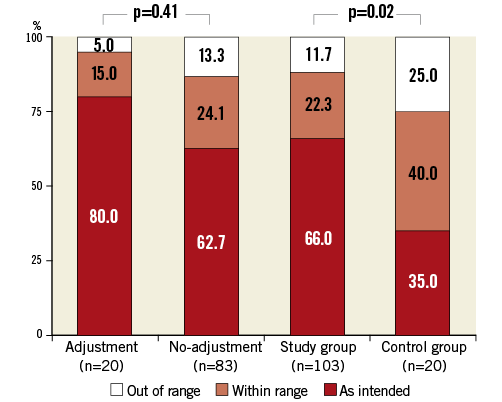
Figure 5. Valve placement class per group.
IMPACT OF VALVE POSITION ADJUSTMENT
For the no-adjustment and adjustment subgroups median deviation of the optimum implantation depth was 7.0% [3.2-15.8] and 5.2% [3.5-9.8], respectively (p=0.37). There was no significant difference in distribution of valve placement class between the subgroups (p=0.41). A trend towards better valve placement could be recognised in the adjustment subgroup, as the incidence of valve placement as intended was considerably higher (80.0 vs. 62.7%; p=0.14) (Figure 4). Valve placement as intended was achieved in 66.7% of the transfemoral (n=8) and all transapical (n=2) and direct aortic procedures (n=6) where adjustments to valve position were made. In the cases of valve position adjustment failure to achieve as intended placement (n=4), overcorrection (too extensive correction resulting in a deviation of valve position from the optimum in the opposite direction) was observed in one patient and undercorrection (too little correction resulting in residual deviation of valve position) in three patients. The case of out of range placement in the adjustment group was due to undercorrection of valve position in a transfemoral procedure.
SHORT-TERM OUTCOME
Short-term outcome is summarised in Table 3. All-cause in-hospital mortality was 2.9% in the study group versus 20.0% in the control group (p 0.03). There were three in-hospital deaths in the study group, all comprising patients in the no-adjustment subgroup. One patient died of haemorrhagic shock due to recurrent haematothorax after otherwise successful transapical TAVI. The second patient, a direct aortic case, died from the deleterious effects of cardiac tamponade followed by sepsis. Both patients had demonstrated good prosthesis functioning on echocardiography. The third in-hospital death resulted from acute cardiac failure due to severe AR caused by delayed valve dislocation, which occurred three days after transfemoral TAVI with out of range too low valve placement (Figure 6A and Figure 6B). An elaborate description of this case can be found in the online supplement.
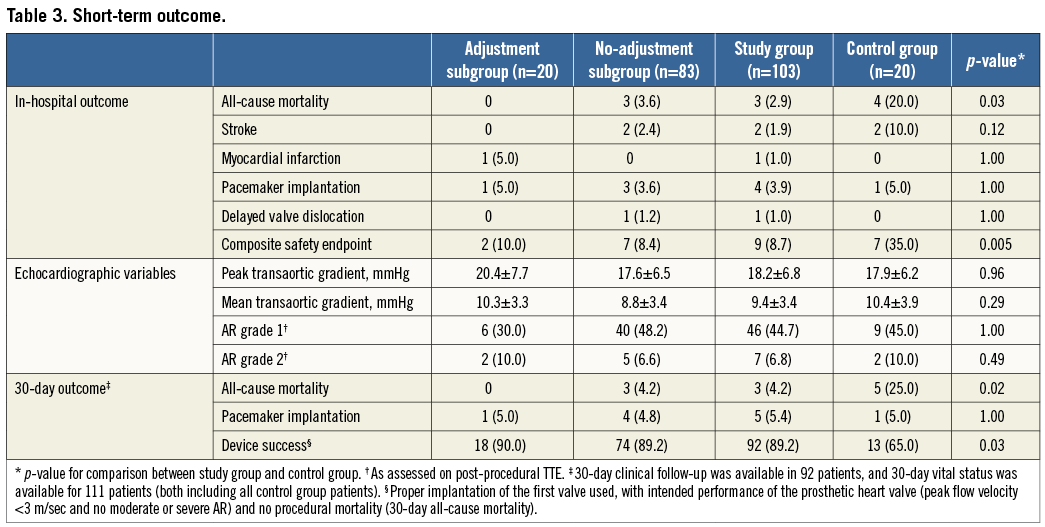
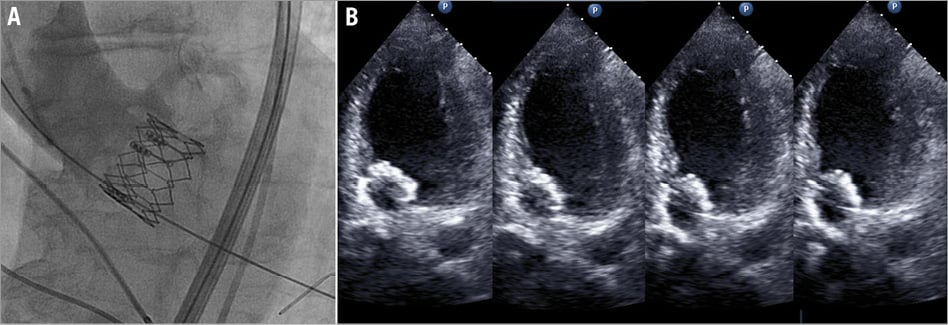
Figure 6. Delayed valve dislocation. A) Final implantation result on angiography demonstrating low valve placement with proper valvular function. B) After acute onset of dyspnoea, echocardiography revealed downward displacement with the prosthesis wiggling in the left ventricular outflow tract along the cardiac cycle, causing obstruction and severe AR.
Stroke was less prevalent in the study group compared to the historical control group, although not statistically significant (1.9 vs. 10.0%; p=0.12). The two cases of stroke observed in the study group (both in the no-adjustment subgroup), comprised ischaemic events in areas supplied by the middle cerebral artery resulting in hemiparesis. One patient improved and was discharged with only minor residual muscle weakness. The other patient had suffered a large severely disabling stroke and was discharged to a hospice for palliative care. Pacemaker implantation rates were comparable, as four patients (3.9%) in the study group and one (5.0%) of the historical controls received a pacemaker due to high-degree AV block. The sole case of myocardial infarction in the study group occurred in a transapical procedure as a consequence of a suture impeding coronary blood flow. There was a significant difference between study and control groups for reaching the composite safety endpoint (9.7 vs. 35.0%; p<0.01).
During 30-day follow-up one additional death occurred in the study group, as the patient who suffered major stroke died due to dehydration. Furthermore, there was one more pacemaker implanted to treat progressive AV block. The composite endpoint for device success was achieved in 92 patients (89.3%) of the study group versus 13 patients (65.0%) in the control group (p=0.03).
Discussion
In this study we evaluated our two-step inflation technique for improving valve placement on a retrospective basis, using a historical control group for comparison. In the following paragraphs the technique itself and the findings on its clinical application will be discussed further.
TECHNIQUE’S RATIONALE
Approximately two years after the start of our TAVI programme we introduced the two-step inflation technique to provide more precision for balloon-expandable valve placement. Higher precision can be achieved by several advantageous means. First, the partially expanded prosthesis provides better reference to the lower sinus border, improving valve position assessment. Second, valve position can still be corrected just before implantation by fine-tuning movements of the valve-delivery system unit. Third, the technique improves predictability of prosthesis behaviour, as operator-independent prosthesis upward movement is diminished and the partially inflated balloon allows for appreciation of flow-mediated balloon movement, all of which can be accounted for when proceeding to definite valve deployment. In combination with the smaller delay between initiation of second-step inflation and prosthesis anchoring, this may reduce the impact of prosthesis movement on final valve position.
FEASIBILITY
The two-step inflation technique is comparable to the technique described by Pasic and colleagues comprising valve deployment under continuous angiographic monitoring, as it also allows for corrections in valve position during deployment7. Importantly, their technique was used in transapical procedures only, whereas our two-step inflation technique was also applied in transfemoral and direct aortic procedures. Our data show that valve position corrections are feasible in all three approaches, albeit performed in a minority of implantations and most frequently in the direct aortic approach. Positioning corrections are often subtle, ranging from just over 1 mm to as much as 4 mm in magnitude.
EFFICACY
Although adjustments were made in only 20% of implantations, the introduction of our two-step inflation technique led to better valve placement compared to historical controls. This emphasises that the technique’s strength not only resides in the potential for valve position corrections, but also in the improvement of the operator’s ability to assess the impact of prosthesis movement during deployment in its final position. The fact that 32 of the 83 procedures in which the operator decided not to change valve position only ended in within range or even out of range placement may be due to the initial reluctance to perform valve position changes, especially in transfemoral procedures. Improper timing of final deployment with respect to prosthesis movement during RVP may also be involved. Notably, valve placement in the control group was generally higher compared to the study group. Previous lack of awareness of the upward phenomenon may have played a role in this. Otherwise, valve placement may have been aimed a little bit higher in the aortic annulus for safety reasons to avoid too low implantation.
Peri-deployment corrections in valve position appeared to be highly efficacious for improving valve deployment, as no fewer than 80% of valve placements in the adjustment subgroup resulted in placement as intended. The best results were achieved in the transapical and direct aortic approaches, most certainly due to the more direct prosthesis control offered by these approaches.
Overcorrection of valve position was not as much of a risk as was undercorrection, since the latter occurred more often and was exclusively responsible for the out of range placement in the adjustment subgroup. All placements not as intended (n=4) in this subgroup occurred in transfemoral procedures, supporting the opinion that correcting valve position is more challenging in this approach. The use of long catheters caused substantial delay in prosthesis response, while the impedance inflicted by tortuous vascular anatomy may produce even less predictable prosthesis behaviour.
SAFETY
Both procedural and clinical outcome suggest that two-step inflation is a safe technique to improve valve placement for balloon-expandable valves. In-hospital and 30-day all-cause mortality were low in the study group as compared to historical controls and in view of recently published figures on TAVI mortality10,11. This may be related to the overall low EuroSCORE in our population, reflecting the large proportion of patients inoperable due to technical constraints. Stroke, pacemaker implantation and moderate AR rates did not differ significantly between the groups. The occurrence of stroke in the study group was in accordance with the incidence recently reported for Edwards SAPIEN implantations in a large international multicentre registry (1.9 vs. 1.7%), whereas the pacemaker implantation rate was lower (3.6 vs. 6.0%)11. These findings may indicate that manoeuvring the partially expanded prosthesis within the calcified aortic valve seems not to increase stroke risk and does not seem to be injurious to the cardiac conduction system. The longer RVP time required for the two-step inflation technique appears not to increase the occurrence of ventricular fibrillation, which was lower than the 13% incidence previously reported12.
The beneficial effect of improved valve placement on clinical outcome remains difficult to substantiate, as complications known to be directly associated with improper placement are rare13. In our cohort there were no cases of coronary artery obstruction or severe AR. One case of valve dislocation occurred in the study group (1.0%), a complication previously observed in 0.4-2.6% of Edwards SAPIEN procedures10,14,15. Despite the absence of indisputable evidence, it seems conceivable that the two-step inflation technique may prevent malpositioning-related complications by improving valve placement.
Limitations
This is a retrospective observational study and therefore subject to all of the shortcomings inherent in retrospective research. There was potential for observer bias, as no blinding was provided for implantation depth measurements. The analysis on valve placement did not account for innovations of the valve delivery system that have increased stability during valve deployment. Amongst these technical refinements are the revision of balloon-inflation geometry resulting in solid anchoring of the prosthesis in the left ventricular outflow tract through expansion at the ventricular side first, and the introduction of new delivery catheters providing tighter balloon-shaft support resisting the inflation forces that tend to push the valve towards the aorta. Furthermore, the group with adjustments in valve position may be regarded as relatively small for thorough safety evaluation of manipulations with a partially expanded prosthesis. Finally, we did not take into account the mandatory lower placement of valves to avoid obstruction of coronary artery ostia in close proximity to the aortic annulus, when grading valve placement.
Conclusions
The two-step inflation technique improves valve placement with respect to implantation depth. The opportunity to correct valve position can be valuable in balloon-expandable TAVI procedures. When corrections are made, valve position is frequently adjusted towards an optimal implantation depth, especially in the transapical and direct aortic approaches. As such, the technique may potentially prevent adverse events due to malpositioning. The two-step inflation technique appears to be safe in this cohort, despite longer rapid ventricular pacing times.
Acknowledgements
The authors would like to thank Astrid Links for her efforts in data collection.
Conflict of interest statement
P. Stella is a physician-proctor for Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Two-step inflation in direct aortic TAVI. The two-step inflation technique used in a direct aortic implantation of a 29 mm Edwards SAPIEN XT prosthesis. The video shows angiographic verification of prosthesis position, followed by fine-tuning adjustments before final deployment.
Appendix. Supplementary methods section
This supplementary methods section provides additional information on the methodology used in the study on the optimisation of balloon-expandable valve deployment by using the two-step inflation technique. This paragraph describes more elaborate patient selection and work-up, the transcatheter implantation procedure and technique, angiographic measurements, study definitions and statistical analysis.
STUDY DESIGN AND PATIENT SELECTION
This is a retrospective single-centre study. All patients who underwent TAVI at the University Medical Center Utrecht using the two-step inflation technique were identified retrospectively. For comparison, a historical control group of 20 patients was selected. Preconditions for patient inclusion in this study were: availability of intraprocedural angiographic images, presence of post-deployment aortographic injection in the same projection used for valve implantation, and no overprojection of procedural material (e.g., wires, catheters, surgical spreaders) obscuring the lower aortic sinus borders.
Patients selected for TAVI were diagnosed with symptomatic severe aortic valve stenosis and considered inappropriate candidates for surgical aortic valve replacement (SAVR). Ineligibility for SAVR was determined by Heart Team consensus and attributable to prohibitive risk for surgery (logistic EuroSCORE >15%) or presence of contraindications (e.g., porcelain aorta, frailty). Further details on patient selection have been published previously16.
The local work-up for TAVI included an outpatient appointment for assessment of physical condition and multimodality imaging. Transthoracic echocardiography (TTE) was performed for evaluation of left ventricular and valvular function, and transoesophageal echocardiography (TEE) for assessment of aortic annulus diameter and concomitant mitral valve disease. Aortic root and annulus dimensions as well as patency of vascular entrance sites were evaluated by thoracoabdominal multislice computed tomography (MSCT). Prosthesis sizing was based on aortic annulus diameter measurements available from TEE and MSCT.
All periprocedural data were retrospectively collected and registered in a database. Follow-up was retrospectively obtained using documentation of standard of care outpatient visits approximately six months following TAVI. All patients gave informed consent for the procedure and, due to the retrospective nature of the study design, ethics committee approval was waived.
IMPLANTATION PROCEDURE AND TECHNIQUE
The balloon-expandable SAPIEN and SAPIEN XT valves (both Edwards Lifesciences Corp., Irvine, CA , USA) were used in this cohort. Both valves consist of a tri-leaflet bovine pericardial valve sewn into stainless steel (SAPIEN) or cobalt-chromium (SAPIEN XT) frame, partially covered with a synthetic fabric sealing cuff.
TAVI was performed by transfemoral, direct aortic or transapical approach, depending on the presence of suitable vascular access. In transfemoral procedures conscious sedation was preferably used; otherwise (in the initial femoral, direct aortic or transapical approach) general anaesthesia was instituted. The majority of transfemoral procedures were assisted by intracardiac echocardiography (ICE), for verification of annular measurements, monitoring of potential complications and assessment of prosthesis function. During the initial femoral procedures and all the non-femoral procedures imaging assistance was accounted for by TEE.
Common access techniques were used for TAVI, as previously described17,18. After establishing access, the procedure continued with identification of the angiographic projection perpendicular to the native aortic annulus. Predilatation was then routinely performed to prepare the device landing zone. The next step was the advancement of the prosthesis towards the aortic annulus for initial placement, verifying the position with angiography (Figure 1A). In transfemoral procedures, initial positioning was done slightly low on purpose, as pulling the system was regarded as safer than pushing it. When valve position was satisfactory, RVP (180-200 bpm) was started, followed by partial inflation of the balloon crimped valve (±1/3 of the full deployment contrast volume) instead of instant complete inflation, the “first step”. Inflation was then interrupted for angiographic reassessment of valve position, which importantly could still be adjusted at that time if deemed necessary for optimisation of implantation depth (Figure 1B and Figure 1C). Finally, when the supposedly desired placement was achieved, the prosthesis was fully deployed by complete inflation of the balloon, the “second step”. All these steps occurred during continuous uninterrupted RVP which would only be stopped after final valve deployment. Following final deployment, prosthesis position and function as well as patency of the coronary ostia were assessed by angiography (Figure 1D). Valvular function was thereafter examined by ICE or TEE in both the short and long-axis views. Additionally, post-dilatation could be performed to address significant prosthetic regurgitation, if incomplete expansion of the prosthesis was suspected. In case of an unsatisfactory result, the decision to implant a second valve was left to the operator’s discretion.
ANGIOGRAPHIC MEASUREMENTS
Angiographic measurements were performed offline using dedicated analysis software (CAAS; Pie Medical Imaging, Maastricht, The Netherlands). Valve implantation depth was measured on angiography in the same projection used for implantation, as the perpendicularity to the annular plane allows for correct appreciation of prosthesis position. Average implantation depth was defined as the average of the distances between the left-sided and right-sided lower sinus border to the most proximal edge of the prosthesis stent frame (Figure 2). To compensate for projection errors, the depth of the prosthesis is expressed as a percentage of total prosthesis stent frame height, as previously proposed19. These percentages were used in combination with manufacturer specified valve heights to calculate implantation depth in millimetres, to give an impression of absolute valve position. All measurements were performed in early systole, as systolic aortic annulus position closely resembles the situation during RVP to which the angiographic projection for implantation is tailored.
DEFINITIONS
The optimal implantation depth for SAPIEN valves used in this study is expressed as a percentage of total stent frame height extending below the lower sinus border on angiography, to enable the use of one single value across the entire range of valve sizes. For evaluation purposes, optimal depth was defined in line with the valve position aimed for during our SAPIEN implantations, namely at 40%. This optimal depth is based on several considerations concerning valve design (height of the fabric cuff) and aortic root anatomy (the proximity of coronary artery ostia and the notion that the “real” aortic annulus is located a little lower than appreciated on angiography). Importantly, the optimal implantation depth definition originates from institutional consensus and lacks scientific evidence for any favourable effect on clinical outcome. Final valve position was arbitrarily classified according to the degree of average implantation depth deviation from the specified optimum, as follows: ≤10% deviation was labelled “as intended”, >10% but ≤20% “within range”, and >20% “out of range” (Figure 3).
To evaluate the efficacy of peri-deployment valve position adjustments, the study group was categorised into “no-adjustment” and “adjustment” subgroups, depending on whether the use of stepwise inflation led to corrections in valve position. These groups were created post hoc, based on a review of all angiographic implantation images by an independent observer, who decided whether operator-driven valve position adjustments were present. For evaluation of technique safety, a composite safety endpoint was created, including mortality, coronary artery obstruction, stroke, pacemaker implantation, myocardial infarction, periprocedural ventricular fibrillation and valve dislocation. Acute procedural success was defined as the successful implantation of a single valve with proper functioning and the patient leaving the operating room alive. Device success was defined according to the VARC-2 criteria.
STATISTICAL ANALYSIS
Data were analysed using IBM SPSS Statistics software version 20 (IBM Corp., Armonk, NY, USA). Continuous variables are presented as means±SD or medians and interquartile range, as considered appropriate, and categorical variables as counts and percentages. For comparison of continuous variables between groups the Student’s t-test or its non-parametric equivalents were used, depending on data distribution. Categorical variables were compared using chi-square or Fisher’s exact test. To account for differences in the study and control groups a propensity analysis was performed by means of binary logistic regression incorporating group membership and the propensity score. The propensity score refers to the propensity to be treated using two-step inflation, and was estimated by non-parsimonious binary logistic regression, including the following (pre)procedural variables: age, gender, length, weight, diabetes mellitus, hypertension, hypercholesterolaemia, chronic obstructive pulmonary disease, pulmonary hypertension, glomerular filtration rate, stroke, peripheral artery disease, abdominal aortic aneurysm, atrial fibrillation, prior pacemaker implantation, acute myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, New York Heart Association functional class, porcelain aorta, frailty, aortic pressure gradient, left ventricular function, procedural approach and the use of general anaesthesia (logistic EuroSCORE was not incorporated because of multi-collinearity). The result of the propensity analysis is reported as an odds ratio (OR) with 95% confidence interval. Two-tailed p-values <0.05 were considered statistically significant.
Case of delayed valve dislocation
The third in-hospital death resulted from acute cardiac failure due to severe AR caused by delayed valve dislocation. The patient concerned was an 83-year-old woman, who received uncomplicated transfemoral TAVI with a 23 mm prosthesis three days earlier. Valve placement was out of range (too low deployment), with an implantation depth 61% of stent frame height. Second valve implantation was considered at the time of implant, but was judged unnecessary as the valve, despite being low, appeared properly “anchored” to the aortic annulus and only trace AR was seen angiographically (Figure 6A). Moreover, ICE showed proper functioning of the valve and there was no paravalvular leakage. On the fourth postprocedural day, the patient experienced sudden onset of dyspnoea and echocardiography revealed downward displacement of the valve prosthesis towards the left ventricular outflow tract causing obstruction and severe AR (Figure 6B). Fast deterioration of the haemodynamic status of the patient occurred leading to death in a few hours (the patient was deemed inoperable by the Heart Team).
Supplementary references

