bstract
Aims: Transcatheter aortic valve implantation (TAVI) is now the therapy of choice for those patients with severe symptomatic aortic stenosis who are considered to be at too high risk for conventional surgery. Balloon aortic valvuloplasty (BAV) is routinely performed to allow placement of the balloon-expandable valve during the procedure. Instrumentation of the valve has been linked to procedural stroke risk, with the associated runs of rapid pacing risking haemodynamic compromise. We outline a novel technique to eliminate BAV prior to transcatheter valve placement.
Methods and results: We illustrate a clinical case that outlines the problems encountered in transcatheter valve placement despite a prior BAV. The solution used in this case involved the partial inflation of the distal section of the balloon allowing easy passage of the SAPIEN XT valve from the transaortic route. After bench testing, we report a series of patients who have undergone this “direct TAVI” procedure from both the transaortic and the transfemoral routes.
Conclusions: In a limited series within a single centre, “direct TAVI” has been shown to be effective in allowing accurate placement of a balloon-expandable device without the need for prior BAV.
Introduction
Transcatheter aortic valve implantation (TAVI) has been shown to be a safe and effective method for the treatment of severe symptomatic aortic stenosis in those patients at prohibitively high risk of conventional surgical valve replacement1. Balloon aortic valvuloplasty (BAV) is usually required prior to deployment of the percutaneous valve due to calcification of the aortic valve apparatus. BAV necessitates a period of rapid pacing, which in itself may be associated with adverse outcome, especially in those patients with poor left ventricular function. There is also some evidence that the highest risk of stroke during TAVI occurs during manipulation of devices in the native aortic valve2. Some investigators have stopped using BAV prior to implantation of a self-expanding TAVI device and have argued that this may potentially reduce the incidence of stroke3.
Our aim is to describe the development of a novel method of deployment of the Edwards SAPIEN heart valve system (Edwards Lifesciences, Irvine, CA, USA) that removes the need for prior BAV.
Methods
We present a series of three cases to illustrate the development of this technique. The first case illustrates the problem encountered when a SAPIEN XT valve would not cross the native aortic valve despite a prior BAV during a transaortic procedure. The second case illustrates an elective use of partial inflation of the valve deployment balloon to facilitate device placement across the stenosed aortic valve, again during transaortic placement. Finally, we present the third case which illustrates the same partial inflation technique but this time via the transfemoral route.
The following is a step-by-step guide to the new technique:
– Advance the whole system to the aortic valve. If the system crosses the valve then proceed as per any balloon-expandable procedure.
– If resistance is felt at the valve, and it is clear that the distal part of the balloon is ventricular, then proceed to inflate the distal part of the delivery balloon. This is achieved by injecting sufficient volume to inflate the balloon without flaring the TAVI device (Figure 1). The usual volume required is 2 mls. Once this is done the inflation device is locked.
– The whole system is then advanced with the distal balloon semi-inflated during a “partial” BAV, thus allowing the device to cross the native valve.
– The pusher is then withdrawn to allow final manipulation of the device into the correct deployment position. Note there is no contrast within the proximal part of the delivery balloon on pusher withdrawal (Figure 1B).
– Finally, the balloon is fully inflated along with rapid pacing to allow device deployment.
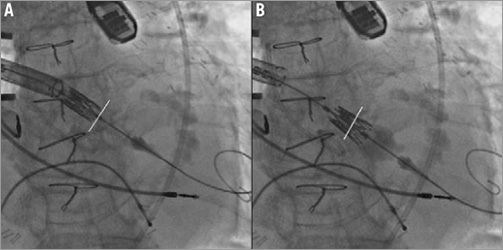
Figure 1. A) Shows the point at which resistance to the valve crossing the aortic annulus is encountered, using a transaortic approach. B) Shows the SAPIEN XT valve crossing the aortic valve annulus following deployment of the distal balloon. The aortic valve annulus is outlined with the white line.
CASE NUMBER 1 (FIGURE 2A, FIGURE 2B, MOVING IMAGE 1)
An 82-year-old male was undergoing transaortic TAVI. Based on preoperative imaging, a 26 mm SAPIEN XT valve was chosen using the Ascendra+ delivery system (Edwards Lifesciences). A BAV was performed with a 20 mm balloon. Despite optimisation of the position of the delivery sheath, wire and device, we were unable to cross the valve with the TAVI device. The Edwards SAPIEN XT was caught on the native aortic valve but it was clear that the distal part of the delivery balloon was across the valve within the ventricle. The patient had no peripheral access to attempt a retrograde BAV. Therefore, we decided to inflate just the distal (ventricular) part of the balloon to facilitate crossing of the valve (Figure 2A). With the pusher element of the introducer sheath fully apposed to the crimped valve, the distal portion of the inflation balloon was inflated using 2 mls of contrast via the Atrion inflation device (Edwards Lifesciences) (Figure 2B, Figure 2C). The inflation device was then locked and the valve successfully deployed across the native valve annulus. After optimal placement, the inflation was continued and the SAPIEN XT valve deployed in the usual manner.
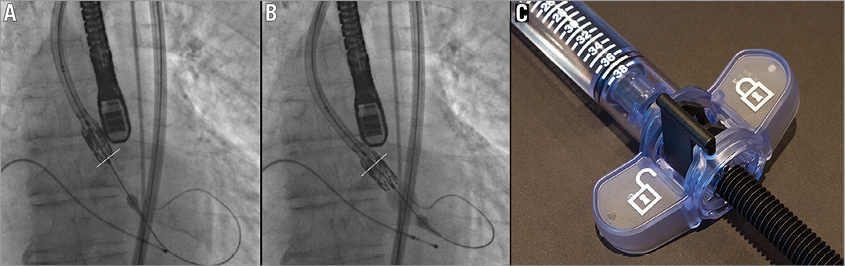
Figure 2. A) & B) Fluoroscopic image illustrating distal balloon inflation to assist in placing the transcatheter valve across the aortic annulus. The aortic valve annulus is outlined with the white line. B) Shows the inflation of the distal section of the balloon using just 2 mls of contrast via the Atrion inflation device (C) which is then placed in the locked position.
BENCH TESTING
After this initial experience we decided to test how the device would react on the benchtop. A SAPIEN XT valve was crimped onto an Ascendra+ system as usual (Figure 3A). Subsequently, volume was placed in the distal part of the balloon, and locked, as per the first clinical case. The distal balloon inflated with no “flaring” of the TAVI device (Figure 3B). The pusher device was withdrawn without difficulty revealing the collapsed proximal section of the balloon (Figure 3C). No movement of the crimped valve was detected on the balloon, and there was no flaring noted on the ventricular side of the valve prior to full deployment.
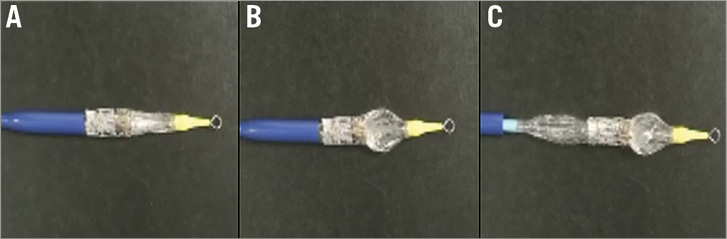
Figure 3. A) Shows the crimped SAPIEN XT valve on the Ascendra+ system prior to balloon inflation. B) Shows the distal balloon inflated, but the pusher fully apposed to the crimped valve. The pusher can then be withdrawn. C) Reveals the fully collapsed proximal section of the balloon, leaving the valve in the crimped position.
CASE NUMBER 2 (FIGURE 1, MOVING IMAGE 2)
In the previous case, the novel technique of partial balloon inflation was used as part of a strategy employed when we encountered difficulty in placing the device across the native valve annulus. We then decided to test this technique as the first strategy used to cross the native valve. The “bail-out” strategy was discussed beforehand: it would have been to withdraw the device into the ascending aorta and perform a retrograde BAV to facilitate crossing of the valve if the new technique were not successful. Case number 2 involved the transaortic placement of a transcatheter aortic valve in an 86-year-old female with patent coronary grafts and severe, symptomatic aortic stenosis. Aortic access was gained through a mini-sternotomy and a stiff guidewire placed through the stenotic aortic valve into the left ventricle. The SAPIEN XT valve was then introduced into the aorta via the introducer sheath.
CASE NUMBER 3 (FIGURE 4, MOVING IMAGE 3)
Case number 3 illustrates that the distal balloon inflation technique can be equally well employed when the vascular access approach is transfemoral. An 86-year-old male with severe, symptomatic aortic stenosis was undergoing transfemoral TAVI and the distal balloon inflation technique was used to allow an atraumatic passage of the SAPIEN XT valve across the heavily calcified native aortic valve.
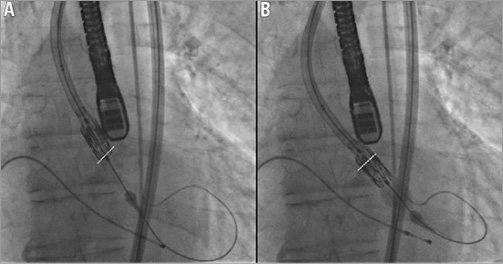
Figure 4. A) Shows the point at which resistance to the valve crossing the aortic annulus is encountered when using the transfemoral approach. B) Shows the SAPIEN XT valve crossing the aortic valve annulus following deployment of the distal balloon. The aortic valve annulus is outlined with the white line.
Results
We have since performed transcatheter valve implantation using this novel direct technique in a series of twelve patients undergoing both transaortic and transfemoral TAVI. Baseline demographics and procedural data are presented in Table 1. Six patients were male, three patients had previously undergone coronary artery bypass grafting, two were in atrial fibrillation and seven had a history of treated hypertension.
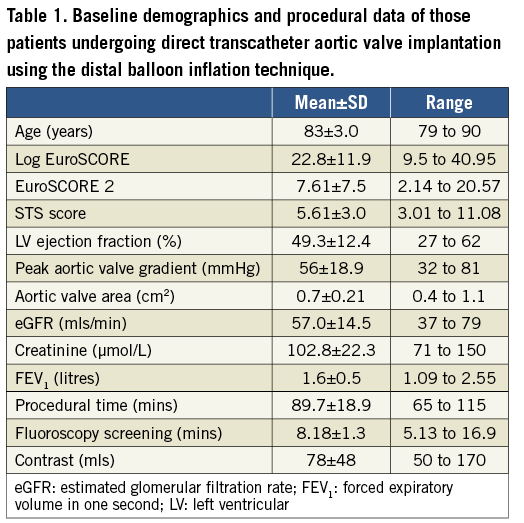
All implants resulted in a successful deployment of the valve. There were no cases of coronary artery obstruction or mitral valve impingement, no post-procedural blood transfusions, and no patient required pacing in the postoperative period. There were no in-patient deaths. One patient suffered a middle cerebral artery ischaemic stroke on postoperative day three, having been neurologically intact on the preceding two days. However, this patient was known to be in atrial fibrillation with a variable ventricular response, and was suboptimally anticoagulated at the time of the event.
Discussion
We describe the development and use of a novel technique for balloon-expandable valve placement. We believe that this technique for deployment of the SAPIEN XT bioprosthetic valve has several potential advantages. By removing the need for BAV prior to valve placement, the risk of temporary but haemodynamically significant aortic regurgitation and thromboembolic stroke may be reduced. Secondly, the staged inflation also allows the valve placement to be more controlled and thus optimal positioning requires fewer manipulations across the annulus. Thirdly, the inflation of the distal balloon may well allow the valve to be placed centrally within the aortic valve annulus, minimising the likelihood of paraprosthetic regurgitation. Finally, the minimisation of rapid pacing runs should reduce the risk of ongoing haemodynamic instability, especially in those patients with poor left ventricular function.
The limitations of the current study are that with low numbers of procedures performed there is the potential that we are yet to encounter a significant complication that may become apparent with greater use. However, were we to be unable to cross the native valve annulus despite partial distal balloon inflation, our bail-out strategy remains the use of a further BAV with the undeployed valve placed in the ascending aorta. We have not encountered any flaring of the crimped bioprosthetic valve with an inflation of 2 mls. However, were the operators to inflate a much greater volume than this, then there would be a risk both of being unable to deploy the valve and of valve displacement from the balloon. Both these complications can be avoided by locking the Atrion device after 2 mls have been injected.
We now no longer routinely perform BAV prior to TAVI deployment. Those cases in which we do are due to concerns as to the aortic valve calcium causing obstruction of the coronary arteries, or in patients with small aortic sinuses leading to a risk of aortic annulus rupture. Our preoperative computed tomography scans and intraoperative transoesophageal echocardiography allow for a much more accurate assessment of annulus size than inflation of the valvuloplasty balloon. We have not seen an increase in the paraprosthetic aortic regurgitation with our technique. In theory, the inflation of the distal balloon could allow the valve to centre within the annulus and may well lead to a more concentric deployment, reducing the risk of paraprosthetic AR.
In conclusion, failure to cross the native aortic valve with a TAVI device is unusual, but important when it happens. We describe a number of techniques to resolve this issue in a non-planned case. We then go on to describe the first planned elective case of “provisional” direct TAVI insertion of a balloon-expandable device using the technique of distal balloon inflation to facilitate crossing of the native aortic valve with the TAVI prosthesis. A subsequent series of twelve patients has proven that, in this limited single-centre experience, the technique appears to be both safe and effective. We believe this will develop into a technique which allows for greater control during deployment and may reduce the rate of post-procedural complications.
| Impact on daily practice We describe a novel technique for the placement of the balloon-expandable SAPIEN XT TAVI valve that removes the need for a prior balloon aortic valvuloplasty. This technique has several potential advantages. These include a decreased risk of haemodynamically significant aortic regurgitation post BAV, a decreased risk of thromboembolic CVA due to fewer manipulations across the calcified aortic annulus, a more central placement of the TAVI valve reducing paraprosthetic AR and fewer pacing runs especially in those patients with a poor left ventricular function. It is now our routine practice to use this technique and omit the pre-deployment BAV during TAVI procedures. |
Conflict of interest statement
M.R. Thomas, C.P. Young, V.N. Bapat and S.R. Redwood are proctors for Edwards Lifesciences Inc. M.R. Thomas and S.R. Redwood have received research funding from Edwards Lifesciences Inc. The other authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Fluoroscopic video illustrating distal balloon inflation to assist in placing the transcatheter valve across the aortic annulus, showing the inflation of the distal section of the balloon using just 2 mls of contrast via the Atrion inflation device, which is then placed in the locked position.
Moving image 2. The point at which resistance to the valve crossing the aortic annulus is encountered, using a transaortic approach. The SAPIEN XT valve crosses the aortic valve annulus following deployment of the distal balloon.
Moving image 3. The point at which resistance to the valve crossing the aortic annulus is encountered when using the transfemoral approach. The SAPIEN XT valve crosses the aortic valve annulus following deployment of the distal balloon.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Fluoroscopic video illustrating distal balloon inflation to assist in placing the transcatheter valve across the aortic annulus, showing the inflation of the distal section of the balloon using just 2 mls of contrast via the Atrion inflation device, which is then placed in the locked position.
Moving image 2. The point at which resistance to the valve crossing the aortic annulus is encountered, using a transaortic approach. The SAPIEN XT valve crosses the aortic valve annulus following deployment of the distal balloon.
Moving image 3. The point at which resistance to the valve crossing the aortic annulus is encountered when using the transfemoral approach. The SAPIEN XT valve crosses the aortic valve annulus following deployment of the distal balloon.

