Abstract
Aims: Many inoperable patients with severe aortic stenosis (AS) are not immediately eligible for transcatheter aortic valve implantation (TAVI). We evaluated the role of percutaneous balloon aortic valvuloplasty (BAV) in this setting.
Methods and results: Among 210 consecutive patients referred to our institution for BAV, we identified three groups: immediately eligible for TAVI (n=65, 31%), excluded from TAVI (n=67, 32%), BAV as a bridge to TAVI (n=78, 37%). This last group comprised patients with low left ventricular ejection fraction, frailty or enfeebled status, symptoms of uncertain origin, critical conditions, moderate-to-severe mitral valve regurgitation, need of major non-cardiac surgery. Outpatient clinic visit and echocardiography were performed around one month after BAV to decide the final therapeutic strategy. Mean age was 81±8 years and the vast majority of patients had comorbidities and high-risk features. The incidence of periprocedural adverse events was 6.4%: 5.1% death (four patients: one procedural complication, three, natural disease progression), 1.3% minor stroke. After BAV, 46% of these patients were deemed eligible for TAVI, and 28% for cardiac surgery. Patients who underwent TAVI after bridge BAV showed 94% 30-day survival.
Conclusions: BAV is a safe and effective tool to bridge selected patients to TAVI when indications are not obvious.
Introduction
Transcatheter aortic valve implantation (TAVI) has recently been shown to be a viable therapeutic option for patients with severe symptomatic aortic stenosis (AS) deemed inoperable, or at very high surgical risk for aortic valve replacement.1-3 However, there are several clinical, functional and anatomic criteria that every patient should meet in order to be a good candidate for TAVI.4
Interestingly, several patients who have been excluded from cardiac surgery exhibit cardiac or extra-cardiac conditions that can also increase TAVI-related morbidity and mortality, such as severely reduced left ventricular function or critical preoperative state, among others. Besides, a relevant proportion of these patients have concomitant comorbidities that could prevent significant postprocedural improvement of clinical status and symptoms relief, such as mitral valve disease, pulmonary hypertension, chronic obstructive pulmonary disease, extreme frailty. In this context, development of strategies to improve patient selection for TAVI would be desirable.
Percutaneous balloon aortic valvuloplasty (BAV) is generally considered a palliative procedure, because restenosis occurs almost invariably in a few months and no clear advantage on survival has been demonstrated.5-7 Nevertheless, relevant, though only temporary reduction of symptoms, amelioration of global clinical status and improvement of echocardiographic parameters are generally observed shortly after the procedure. For these reasons, BAV could be used either as a bridge to TAVI in patients at high risk of periprocedural complications, or as an additional selection tool whenever there are clinical doubts about the indications to TAVI. With this study, we sought to evaluate a possible role of BAV in patients’ selection for TAVI.
Methods
Patient population
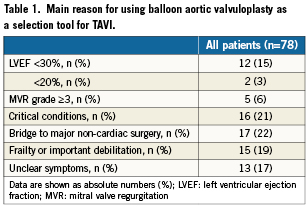
Between July 1st 2007 and December 31st 2009, 210 patients with severe symptomatic aortic stenosis were referred to our institution for BAV. They were all screened for TAVI based on clinical and anatomic characteristics.4 Demographics and baseline clinical and functional characteristics were prospectively collected in a dedicated database. After careful evaluation of indications and contraindications to TAVI, 65 patients (31%) were deemed eligible to TAVI, 67 (32%) were definitely excluded from TAVI, and 78 patients (37%) received BAV as a bridge to TAVI. Classification of the patients required consensus of at least two physicians. Patients were definitively excluded from TAVI and received only palliative BAV for the following reasons: anatomic criteria precluding TAVI with both the available devices (Edwards SAPIEN™ balloon expandable valve [Edwards Lifesciences Inc., Irvine, CA, USA] and the self-expandable Medtronic CoreValve Revalving System™ [Medtronic, Minneapolis, MN, USA]), mainly pertaining to the size of the aortic annulus, and the availability of a site of access (aorto-ilio-femoral axes, subclavian arteries, left ventricle apex), other terminal illness, and dementia. Patients who received BAV as a “bridge” to TAVI comprised patients who were possible candidates for TAVI but, at the time of BAV, showed either a temporary contraindication to the procedure or presented clinical/echocardiographic characteristics suggesting further evaluation before a definitive therapeutic decision could be taken. Patients included in this group must have high-risk for surgical aortic valve replacement plus at least one of the following: low left ventricular ejection fraction (LVEF<30%); important frailty or enfeebled status (qualitative assessment); symptoms of uncertain origin (i.e., dyspnoea in patients with concomitant severe chronic obstructive pulmonary disease); critical conditions (acute pulmonary oedema, cardiogenic shock); moderate-to-severe mitral valve regurgitation; bridge to major non-cardiac surgery (Table 1). All these patients underwent an outpatient clinic visit around one month after BAV, and echocardiography if deemed necessary, in order to select the final therapeutic strategy.
Balloon aortic valvuloplasty
All procedures were performed through the femoral artery using the standard retrograde technique. The Cristal Balloon™ (Balt, Montmorency, France) was used in all cases. In our centre, a “moderately aggressive” BAV technique is commonly used with the rationale that BAV is mainly aimed at short- to mid-term symptom relief. For this purpose, moderate increase of aortic valve area (AVA) is usually considered sufficient and any attempt to further improve haemodynamic results is associated with enhancement of risks. Unless the patient had a very small (<18 mm) or a very large (>25 mm) annulus size assessed with echocardiography and/or angiography, a 20 mm balloon was used and eventually upgraded only if deemed strictly necessary by the operator. One to three manual inflations were made until abrogation of aortic pulse was achieved for a few seconds. We aimed to obtain a reduction of 50% of the initial gradient and/or an increase ≥0.2 cm2 of AVA. We did not employ rapid pacing during balloon valvuloplasty with a very few exceptions, that is, when a stable position of the balloon could not be obtained without rapid pacing. The amount of contrast medium was kept at a minimum, and in patients with severe renal impairment we did not use contrast at all. Haemostasis was accomplished using the 8 Fr Angio-Seal haemostatic device (St. Jude Medical, St. Paul, MN, USA), application of a compressive bandage, and bed rest for 24 hours.
Definitions and endpoints
Severe aortic stenosis was defined as aortic valve area (AVA) <1 cm2 and AVA indexed <0,6 cm2/m2. Symptomatic status was classified based on the presence of syncope, stable angina (graded according to Canadian Cardiovascular Society classification), acute coronary syndrome, dyspnoea (New York Heart Association classification), and cardiogenic shock. Peripheral vascular disease included a history of intermittent claudication, previous peripheral vascular surgery, or documented peripheral arterial stenosis greater than 70%. Cerebrovascular disease was defined by previous stroke or transient ischaemic attack. Chronic renal insufficiency was considered a glomerular filtration rate <60 ml/min calculated by the Cockcroft-Gault formula. The diagnosis of anaemia was made if serum haemoglobin was <13 g/d for men and <12 g/dl for women. Severe chronic obstructive pulmonary disease was identified by a forced expiratory volume in 1 second (FEV1) <1 litre or long term use of bronchodilators, steroids or oxygen for lung disease. Cardiogenic shock was characterised by systolic blood pressure <90 mmHg with signs of low peripheral perfusion or necessity to administer inotropes for circulatory support.
We assessed the incidence of the following events: death, myocardial infarction, stroke, severe acute aortic regurgitation (SAAR), access-site related vascular complications (retroperitoneal bleeding, pseudoaneurysm, arterial-to-venous fistula, arterial dissection). The follow-up was performed with different sources: telephone interviews with the patients or their relatives, outpatient clinic, hospital discharge records, municipal civil registries of mortality.
Statistical analysis
Continuous variables were expressed as mean±standard deviation and were compared using Student’s unpaired t-test. Categorical variables are presented as numbers and percentages and chi-square test was used for comparison. Cumulative event rates were estimated by the Kaplan–Meier method and compared by the log-rank test. Patients were censored at the time of the last contact. All analyses were performed with the SPSS 15.0 software (SPSS Inc., Chicago, IL, USA).
The study was conducted according to the Declaration of Helsinki. The local medical ethics committee approved the protocol and written informed consent was obtained from every patient.
Results
Baseline characteristics of the population are reported in Table 2. Patients clearly exhibit high-risk features, as evidenced by advanced age (mean 81.1±8.2 years), high logistic EuroSCORE (22±13%), and the presence of several comorbidities. Overall, 41% of the patients had coronary artery disease, 10% had previous cardiac surgery, and 8% previous cerebrovascular accidents. Three patients (4%) presented with cardiogenic shock.
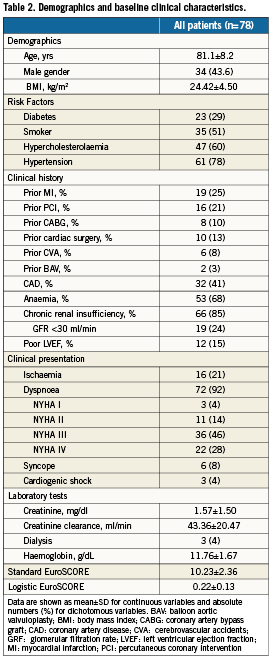
At echocardiography (Table 3), mean AVA was 0.65±0.13 cm2, mean transvalvular gradient 49±17 mmHg, and LVEF 53±16%. A 20 mm balloon was used in 73 (93.6%) patients, 18 mm in three (3.8%), and 23 mm in two (2.6%). In one patient a 20 mm balloon was used after the 18 mm to improve the procedural result. Echocardiographic parameters following BAV are also reported in Table 3. There was an average increase of AVA of 0.29±0.31 cm2.
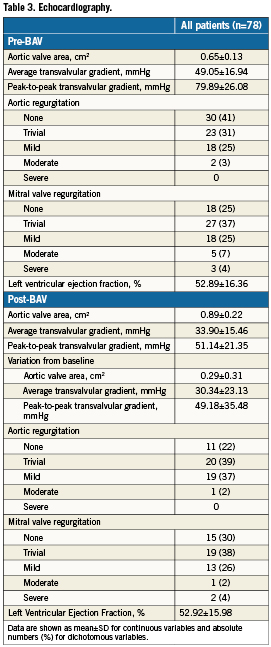
The overall incidence of periprocedural adverse events was 6.4% and was as follows: four (5.1%) in-hospital deaths (two in the critical condition subgroup, one each in the low LVEF and in the frailty subgroups) and one (1.3%) minor stroke with full recover within 24 hours. No patient had acute myocardial infarction and one patient developed SAAR successfully managed in the catheterisation laboratory; echocardiography post procedure showed residual moderate regurgitation. There were no cases of cardiac tamponade, atrioventricular block, coronary occlusion, aortic rupture. Similarly, there were no access-site related vascular complications nor significant bleedings. In reviewing the causes of death, three out of four fatal events could be attributed to disease progression (no efficacy of the BAV) rather than to procedural complications. The other death occurred because of mesenteric artery embolisation post-BAV in a patient with acute pulmonary oedema not responding to full medical treatment. In this perspective, the true incidence of procedural complications was 2.6%.
All surviving patients were re-evaluated one month after the procedure and the result of the second evaluation is showed in Figure 1. Overall, 36 patients (46%) were finally accepted for TAVI, and 22 (28%) were even deemed eligible for surgical AVR. Sixteen patients (21%) did not exhibit any improvement and were addressed to medical therapy.
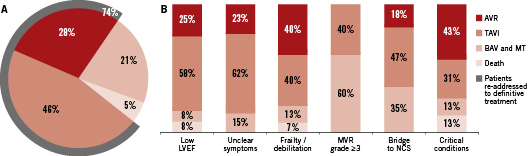
Figure 1. Allocation of patients following re-evaluation one month after “bridge” balloon aortic valvuloplasty (BAV). A. All patients. B. Proportion of patients re-allocated to the different treatment strategies after BAV in the different subgroups. AVR: aortic valve replacement; LVEF: left ventricular ejection fraction; MT: medical therapy; MVR: mitral valve regurgitation; NCS: major non-cardiac surgery; TAVI: transcatheter aortic valve implantation
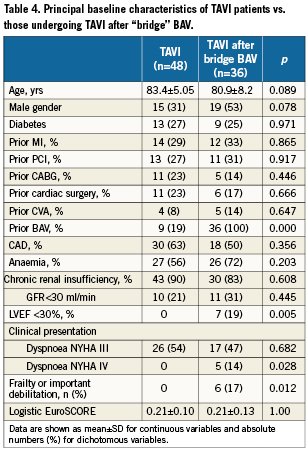
In order to confirm the validity of our strategy, we compared the 30-day survival following TAVI of the patients selected after “bridge” BAV with the outcome of the first 47 consecutive patients undergoing TAVI in our institution who were immediately eligible to TAVI. As showed in Figure 2, no significant difference was observed between the two groups, despite the worse baseline profile of the bridge cohort (Table 4). Remarkably, thus far 11 of the 22 patients re-addressed to AVR have undergone cardiac surgery with no in-hospital deaths. Patients who underwent BAV and continued medical therapy only were all alive at 30 day follow-up, and all but one presented some degree of functional improvement, confirming the palliative value of BAV at least in the short-term.
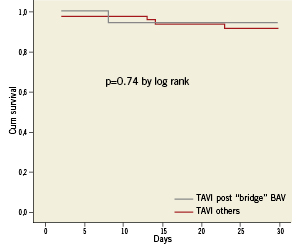
Figure 2. Kaplan-Maier 1-year survival curves following transcatheter aortic valve implantation (TAVI) in patients initially attributed to “bridge” BAV as compared to 47 consecutive other patients who underwent TAVI in our hospital.
Discussion
The principal findings of this study can be summarised as follows: 1) BAV is a useful tool to categorise patients with severe symptomatic aortic stenosis who have been denied surgical AVR when indications to TAVI are not obvious; 2) The use of BAV is associated with rapid clinical and/or functional improvement allowing eligibility of around ¾ of these patients for definitive invasive treatment, including surgical aortic valve replacement in 28%; 3) BAV is relatively safe in patients with high-risk features.
BAV was originally conceived as an alternative to AVR in patients with severe symptomatic AS.8 However, after the initial enthusiasm, the interest in BAV waned quickly because of early recurrence of stenosis and the alleged inability to change the natural course of the disease,5-7 coupled with high rates of acute complications.9,10 Until recently, BAV has therefore maintained only a small niche as a palliative procedure or as a bridge to AVR in very selected patients.11,12 The interest toward BAV has now flourished again thanks to the development of TAVI. This renewed interest, however, found its basis in the use of the technique as a preliminary step integrated with the TAVI. With this study we aimed to demonstrate another possible utilisation of BAV, i.e., as a tool for patient selection before TAVI. The fact that a considerable proportion of patients with severe symptomatic aortic stenosis are denied surgical intervention is not new.13-16 Conversely, fairly new as well is the information that several patients referred for TAVI might not be suitable for this procedure either.4 In the present series of patients excluded from AVR, we documented that ineligibility to TAVI could be either permanent (32% of the patients) or temporary (37%). In these last patients, we demonstrated that “bridge” BAV is an excellent option to re-allocate to definitive treatment after an additional short-term evaluation. The rationale behind that is that BAV is associated with clinical improvement of critically ill patients, LV function recover and/or reduction of mitral valve regurgitation, and recover from severe debilitation in around 75% of the cases. It should be acknowledged that reduced left ventricular function and frailty are generally considered reasons to address patients directly to TAVI. However, some of our patients had a severely compromised LVEF i.e., <20%, that was also an exclusion criteria for TAVI in the Placement of Aortic Transcatheter Valves (PARTNER) trial.1 The LVEF<30% criteria was, instead, the initial screening criteria applied in the proctored phase of commercialisation of the transcatheter aortic valves and, although debatable, it is still applied in many centres. Frailty is a quite elusive definition, and certainly some fragile patients are immediately eligible for TAVI. Nevertheless, there are some patients so fragile and debilitated that, in our opinion, every aggressive therapeutic strategy should be seriously weighted against expected benefits and in this scenario a “bridge” BAV can be useful. In an even larger proportion of patients with unclear symptoms, we suggest that BAV enhances the chances of predicting functional improvement after TAVI. This last aspect is not trivial, given the very high cost and the risks associated with a TAVI procedure. Most obvious, but not irrelevant, is the observation that BAV might be used as a bridge to TAVI in patients who need major non-cardiac surgery and, for different reasons, are not candidates for subsequent AVR. Interestingly, in perfect agreement with our findings, a recent study raised the hypothesis that staging BAV before TAVI may be an effective option for reducing the rates of complications in patients at high risk,17 based on the assumption that the results of TAVI are strongly influenced by the selection strategy, and unstable patients are more likely to have a critical early post-operative period. The 30-day survival of our “bridge” cohort of patients was, in fact, similar to that of other patients who underwent TAVI at our centre, despite the fact that a worse survival might have been expected.
It is noteworthy that 28% of the patients undergoing a second clinical evaluation after BAV were finally accepted for AVR, and no complications arose in the peri-operative phase in those who actually underwent the operation. This confirms previous observations that a sizable proportion of the patients referred for TAVI procedure could finally undergo surgical AVR with a good outcome,18,19 highlighting the opportunity of what we call a “dynamic” multidisciplinary evaluation in tertiary centres with capabilities for both percutaneous and surgical interventions.
Finally, with this report we challenge the widespread belief that BAV is associated with high rates of serious complications.9,10 All large registries of BAV procedures have been performed around 20 years ago and an update of procedural-related complications would be appropriate. Despite the fact that no major technical changes have occurred, use of smaller catheters and vascular closure devices for arterial haemostasis might be associated with a lower incidence of vascular complications. Severe acute aortic regurgitation is rare, and most of the time is caused by the immobilisation of a cusp in the opening position, which can be more often corrected with relatively simple manoeuvres. Indeed, low rates of periprocedural complications in high-risk populations have been reported in other small recent series as well,17,20,21 As previously described, we employ a “moderately aggressive” BAV technique, meaning utilisation of balloons often undersized without pursuing the achievement of excellent haemodynamic results. In our opinion, this markedly reduces the chances of acute complications to occur without compromising much the short-term clinical results. This may be particularly true for the patients representing the focus of the present study, i.e., those that underwent a second evaluation around one month after the procedure to select the final therapeutic strategy. It is noteworthy that all in-hospital deaths in this group occurred in really critical patients; only in one case was there a true procedure-related complication (embolisation to the mesenteric artery), which simply accelerated a foretold death.
This analysis is subject to the limitations of observational studies. These results represent the experience of a single high-volume centre and may not be widely generalised. Clearly, the acceptance rate for TAVI could be different between different centres. However, availability of both commercial devices and experience with all presently available access routes (percutaneous, trans-subclavian, transapical) made our inclusion criteria quite broad, at least from the anatomic standpoint. Overall, taking into account the 36 additional patients selected after “bridge” BAV, 101/210 patients (48%) were finally accepted for TAVI, which is consistent with other experiences reported in the literature. Attribution of the patients to the “bridge” group was based on arbitrary criteria, with the principal aim to provide a “proof of concept” instead of identifying clear-cut criteria suggesting BAV before TAVI.
Conclusion
BAV represents a useful tool for evaluating patients with severe aortic stenosis that have been previously excluded from surgical aortic valve replacement, but have no clear indications to TAVI. In these patients, BAV can be performed safely and may work as a bridge to definitive invasive treatment.
Acknowledgement
We would like to thank Dr. Valentina Ovi for her precious help in data collection and monitoring.
Conflict of interest statement
The authors have no conflicts of interest to declare.

