Abstract
Aims: To describe a technique of simultaneous aortography and balloon aortic valvuloplasty (BAV) before transcatheter aortic valve replacement (TAVR), and to show how this technique affected TAVR prosthesis selection and procedural outcomes.
Methods and results: One hundred and eleven patients underwent simultaneous contrast injection during valvuloplasty pre-TAVR to confirm the indication for prosthesis size provided by non-invasive imaging studies. A successful injection was achieved in 95 patients (85.5%). No events occurred during simultaneous BAV and contrast injection. In 12 (10.8%) patients the prosthesis size implanted was different from the recommendations provided by the non-invasive imaging examinations. In nine of these cases (75.0%) it was decided to implant a larger prosthesis than that originally suggested, in the remaining three cases (25.0%) a smaller valve was implanted. Device success in this particular subset of patients was 100%. Overall device success was 92.8%. Post-procedural moderate paravalvular regurgitation was reported in 5.4% of patients.
Conclusions: In patients with severe aortic valve stenosis, a technique of simultaneous aortography and balloon valvuloplasty as an adjunct to non-invasive imaging modalities for transcatheter prosthesis selection is feasible, and leads to a change in TAVR strategy in a modest number of patients. Larger studies are necessary to confirm these findings, and to assess whether this method is capable of enhancing the safety of the TAVR procedure.
Abbreviations
AS: aortic stenosis
BAV: balloon aortic valvuloplasty
CV: CoreValve
ES: Edwards SAPIEN
Fr: French
MSCT: multislice computed tomography
PVR: paravalvular regurgitation
STS: Society of Thoracic Surgeons
TAVR: transcatheter aortic valve replacement
TTE: transthoracic echocardiography
TEE: transoesophageal echocardiography
VARC: Valve Academic Research Consortium
Introduction
Transcatheter aortic valve replacement (TAVR) can now be considered an effective treatment for patients with severe aortic stenosis (AS) at high risk for conventional aortic valve replacement1. The success of the procedure is closely related to the correct choice of prosthesis size. Incorrect sizing may result in adverse outcomes such as paravalvular regurgitation (PVR)2, device embolisation3 or even aortic root rupture in the case of severe oversizing4. In recent years, multislice computed tomography (MSCT) has emerged as the most valuable tool in providing reproducible annular measurements, and in predicting significant paravalvular leak after TAVR5. However, MSCT has a margin of error, particularly in borderline annuli, is often denied in patients with impaired renal function, and is not able to simulate the presence of a prosthetic valve into the aortic root, thus reproducing the movement of the calcified cusps in relation to the aortic walls and the coronary ostia.
The aim of this pilot study was to describe a technique of simultaneous contrast injection into the sinus of Valsalva during balloon aortic valvuloplasty (BAV) pre-TAVR, and to show how this technique affected prosthesis selection and procedural outcomes.
Methods
PATIENT POPULATION AND DEVICE
Between June 2007 and July 2012, among a total of 320 patients who underwent TAVR with the 18 French (Fr) self-expanding CoreValve (CV) prosthesis (Medtronic Inc., Minneapolis, MN, USA) and the balloon-expandable Edwards SAPIEN (ES) XT heart valve (Edwards Lifesciences, Irvine, CA, USA), 111 (35.1%) underwent simultaneous aortography during valvuloplasty pre-TAVR to confirm the indication for prosthesis size provided by non-invasive imaging studies.
The design features of the CV and ES prostheses and technical details of the procedures have been described previously6,7. The CV prosthesis, available in 26 mm and 29 mm sizes, and from September 2011 also in 31 mm size, was implanted using either the transfemoral or the subclavian approach with an 18 Fr delivery catheter, later improved by an AccuTrak Stability Layer (Medtronic Inc.). The ES prosthesis, available in 23 mm and 26 mm sizes only, was implanted by means of the transfemoral approach using the Retroflex 3 or NovaFlex delivery catheters (Edwards Lifesciences). All procedures were performed under local anaesthesia (with or without additional sedation and/or analgesia), under fluoroscopic guidance, in a standard cardiac catheterisation laboratory with surgical back-up. All patients and their close relatives signed written informed consent.
PATIENT SELECTION
Screening studies were performed in all patients before the procedure. These included transthoracic echocardiography (TTE) or, occasionally, transoesophageal echocardiography (TEE), angiography (ascending aorta, coronary arteries, and iliofemoral vessels), and MSCT. During the early experience, prosthesis size selection was ordinarily performed by using TTE, TEE and MSCT, according to a single-plane annular measurement. Later, this was mostly done by measuring the area-derived diameter and basal ring average diameter with MSCT.
CONTRAST DYE INJECTION DURING BAV
All patients had BAV under rapid pacing (160-200 bpm) before transcatheter heart valve implantation. The indications for performing simultaneous aortography and BAV were: a) borderline aortic annulus for small or large valve size as assessed by echo or MSCT; b) suboptimal quality of the non-invasive imaging examinations; c) anatomical features of the aortic root potentially suggesting a high risk of coronary occlusion or annular injury by the calcified aortic cusp (i.e., narrow sinus of Valsalva, low origin of the coronary ostia, or bulky leaflet calcification).
Technically, once all the three aortic cusps had been aligned in the same plane, the balloon valvuloplasty with a medium compliance balloon (with a known amount of contrast in order to achieve a known pre-measured diameter) was performed during rapid pacing in a standard fashion (Figure 1). The pigtail catheter was positioned at the level of the aortic annulus, or slightly above the balloon if the sinotubular junction was wide enough to allow the whole dyeing of the sinuses of Valsalva. Fifteen mL of contrast was injected and the rate of injection was ≥10 cc/s at 900-1,100 pounds per square inch. The injection was considered successful when the sinuses of Valsalva became clearly visible. Besides guiding the choice of prosthesis size, the patency of the left main and the movement of the calcified cusps toward the walls of the sinuses were also assessed while the balloon was entirely inflated. The BAV catheter was selected so that the diameter of the balloon was 1-3 mm smaller than the prosthesis selected according to the measurement of TTE, TEE or MSCT; balloon lengths were 4, 4.5, or 5 cm. The balloons used were the Z-MED II and the Nucleus™ balloons (NuMed, Inc., Hopkinton, NY, USA), and the Cristal balloon (Balt Extrusion, Montmorency, France).
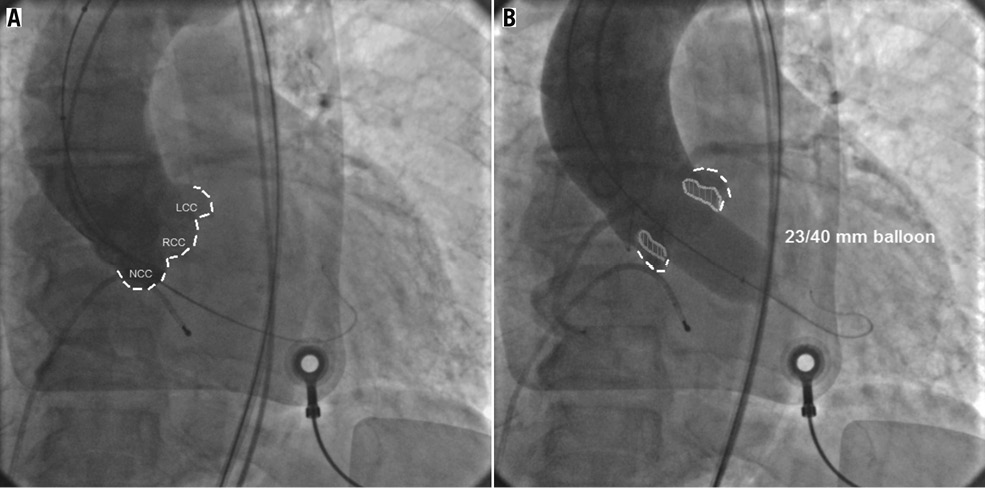
Figure 1. A) Aortography showing the three coronary cusps (dotted line) aligned on the same plane. A stiff guidewire is placed into the left ventricle. B) An aortic valvuloplasty is performed using a 23/40 mm Z-MED II balloon (NuMed, Inc.) during rapid pacing (180-220 bpm) and contrast dye is injected into the aorta through a standard pigtail catheter. In this case neither regurgitation of contrast dye into the left ventricle, nor threatening movement of the calcified cusps (depicted in grey) toward the aortic root walls or the left coronary ostia can be seen. A 26 mm Edwards SAPIEN XT valve was implanted uneventfully. LCC: left coronary cusp; NCC: non-coronary cusp; RCC: right coronary cusp
DEFINITIONS AND STATISTICAL ANALYSIS
Cardiovascular death, periprocedural and spontaneous myocardial infarction, strokes, bleedings and vascular access-site and access-related complications and echo outcomes were defined according to the Valve Academic Research Consortium II (VARC-II) definitions8. Continuous variables are presented as mean±standard deviation and were compared using the Student’s unpaired t-test or the Mann-Whitney rank-sum test, based on a test for normal distribution. All data were processed using the Statistical Package for Social Sciences, version 17 (SPSS Inc., Chicago, IL, USA).
Results
PATIENT CHARACTERISTICS
The baseline demographic and clinical variables of the patients are presented in Table 1. Overall, the population was at high surgical risk with a predicted mortality of 7.6±6.1% by the STS mortality risk score, and had severe symptomatic AS (mean aortic valve area [AVA] 0.59±0.1 cm2, mean gradient 51.6±14.2 mmHg). The majority of patients (n=80; 72.1%) were in NYHA Functional Class III or IV before the procedure. Transfemoral access was used in 110 patients (99.1%); in one patient (0.9%) where the transfemoral approach was unfeasible, a trans-subclavian access was used to implant a CV device.
ANGIOGRAPHY AND BAV
Overall, a total of three different balloons were used: the Nucleus balloon in 57 cases (51.3%); the Z-MED II balloon in 50 cases (45.0%), and the Cristal balloon in four cases (3.7%). The calibre of all balloons was measured prior to use. The mean balloon diameter was 22.3±1.7 mm (range 20-25 mm). A successful injection was achieved in 95 patients (85.5%) after a mean number of inflations of 1.34±1.6 (range 1-3). In the vast majority of cases (n=82; 73.8%) a single BAV and simultaneous contrast injection was performed, whereas in 21 (18.9%) and eight (7.2%) cases, two and three attempts were made, respectively. Of note, an unsuccessful procedure was associated with a higher number of inflations (1.94±0.68 vs. 1.23±0.5; p<0.001). The majority of unsuccessful injections were caused mostly by the incorrect position of the pigtail catheter or by the instability of the balloon during the inflation. Also of note, a significant reduction in the number of inflations to achieve an adequate aortic root dyeing and a higher successful injection rate were reported over time (Figure 2). In addition, a higher rate of successful injection was reported when a 4.0 cm balloon catheter was used compared to a 4.5 or 5.0 cm balloon (93.0% vs. 60.0%; p<0.001). No events occurred during simultaneous BAV and contrast injection. After BAV, no cases of massive aortic regurgitation requiring haemodynamic support were reported.
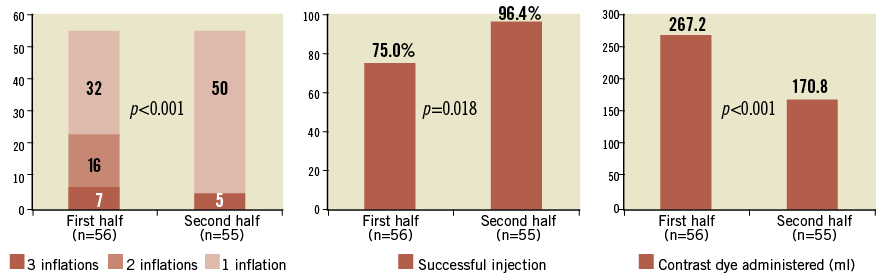
Figure 2. Bar charts illustrating: A) the impact of a learning curve on the number of inflations to achieve an adequate dyeing of the aortic root; B) the rate of successful injections; C) the amount of contrast dye administered.
TAVR PROCEDURAL OUTCOMES
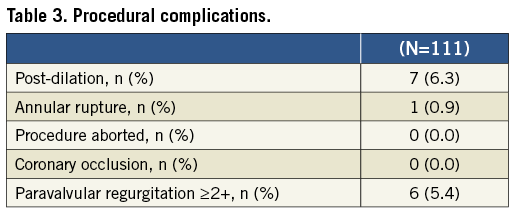
The main procedural variables are presented in Table 2. The CV prosthesis was used in 64 patients (57.6%) whereas the ES valve was implanted in 47 patients (42.4%). Procedural complications are listed in Table 3. The VARC-modified device success rate was 92.8%. The incidence of moderate PVR as assessed by TTE intraprocedurally was 5.4%. Post-implant dilatation due to prosthesis underexpansion was used in 6.3% of patients. Noticeably, a case of annular rupture creating an aortic-right atrium fistula (case #4) due to a bulky calcification located in the right cusp was reported after 26 mm ES implantation (size recommended by MSCT and confirmed by the aortography during BAV). No cases of coronary occlusion by a calcified cusp were reported.
IMPACT OF SIMULTANEOUS BAV AND ANGIOGRAPHY ON TAVR STRATEGY
Overall, in 12 (10.8%) patients who underwent simultaneous contrast injection during BAV the choice of the prosthetic size was different from the recommendations provided by the non-invasive imaging exams (Table 4). Of these, in two patients MSCT was not performed because of severe renal dysfunction, and in four early patients prosthesis size choice was not achieved by measuring the area-derived diameter and basal ring average diameter. In nine cases (75.0%) it was decided to implant a larger prosthesis than that originally suggested, because the injection revealed the presence of regurgitation into the left ventricle while the balloon was fully inflated (balloon/measured annulus ratio 1:1) (Figure 3). In the remaining three cases (25.0%) a smaller valve was implanted: the angiography showed that the sinuses of Valsalva were not wide enough to host the bulky calcifications of the leaflets (Figure 4). Among all these patients, neither moderate PVR nor procedural valve-related complications was reported. Finally, in two patients a coronary wire was placed in the distal left anterior descending before TAVR, because, despite the acceptable height of the left main, angiography displayed a large calcification on the left coronary cusp moving toward the left main ostia during balloon inflation. In these cases, it was decided to deploy the ES prosthesis slightly more ventricular in order to minimise the presence of the stent into the sinuses. After the ES prosthesis implantation no stenting was required. PVR was trace in both patients.
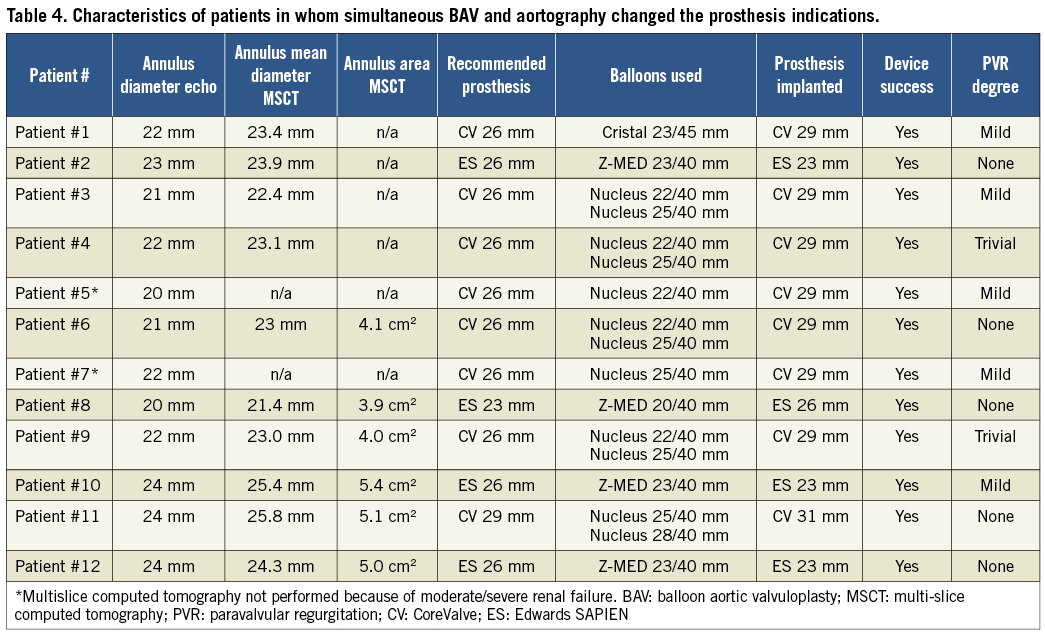
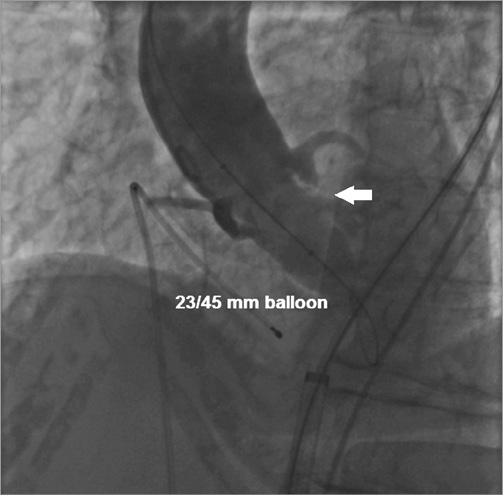
Figure 3. Aortic valvuloplasty performed with a 23/45 mm Cristal balloon (BALT Extrusion, Montmorency, France) in a patient in whom implantation of a 26 mm CoreValve prosthesis was suggested by transoesophageal echocardiogram (annulus 22 mm). Simultaneous injection revealed the presence of regurgitation into the left ventricle while the balloon was fully inflated (white arrow). In this case it was decided to implant a 29 mm CoreValve prosthesis with good final results.
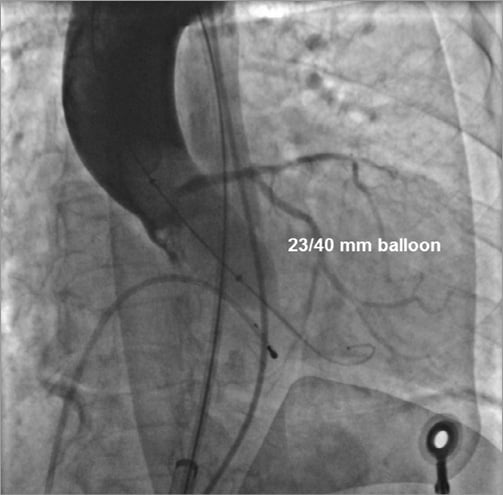
Figure 4. Aortic valvuloplasty performed with a 23/40 mm Z-MED II balloon (NuMed, Inc.) in a patient in whom implantation of a 26 mm Edwards SAPIEN XT prosthesis was suggested by computed tomography (area 5.0 cm2). Simultaneous angiography showed that the sinuses of Valsalva were not wide enough to host the bulky calcifications of the cusps; therefore, it was decided to implant a 23 mm Edwards SAPIEN XT prosthesis with good final results.
TAVR OUTCOMES
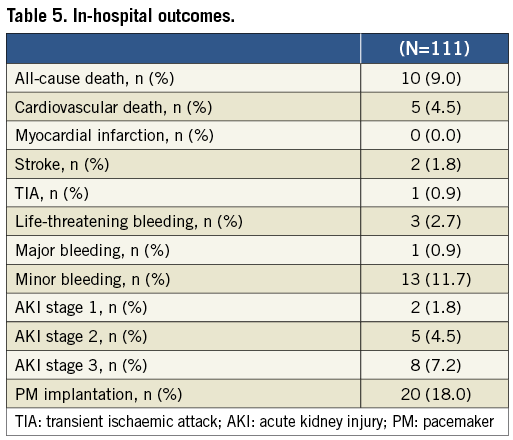
Overall, in-hospital all-cause mortality and cardiovascular mortality were 9.0% and 4.5%, respectively. In-hospital outcomes are presented in Table 5.
Discussion
In this pilot study we described a technique of simultaneous contrast injection into the sinuses of Valsalva during BAV pre-TAVR to confirm the indications for device size provided by non-invasive imaging modalities. In relation to all the traditional imaging modalities, the uniqueness of this method is that it provides a dynamic visualisation of the movement of the cusps and their relationships with the aortic root while the balloon is inflated. This aspect is extremely important for TAVR, since the biological prosthesis does not replace the native valve, but is deployed inside it. As far as we know, this is the first systematic evaluation of this method for selecting prosthesis size in patients undergoing TAVR. Recently, Babaliaros and co-workers described a strategy for selecting transcatheter heart valve size based upon aortic annulus measurement during BAV in 27 patients. According to the results of this study (neither major complications nor PVR more than mild, and the finding that in 26% of patients balloon sizing resulted in selection of a specific prosthesis size that could not be achieved by TEE alone), they concluded that such a technique may improve the safety and efficacy of TAVR9. Compared with this latter technique, the one described in this analysis incorporated a simultaneous aortography to assess both the appropriateness of the indication given by MSCT or echo, and the potential risk of annular injury or coronary occlusion during valve deployment. Consequently, several important points can now be made.
TECHNICAL ASPECTS AND SAFETY
First of all, the technique is feasible and provides good visualisation of the sinuses of Valsalva in more than 85% of cases. This is obtained with no additional risks of kidney failure or overt neurological events, compared with previous TAVR reports. Furthermore, it might be argued that such a strategy compels the prosthesis to be loaded only after BAV. However, no cases of severe aortic regurgitation after BAV were reported among the 111 procedures. An unstable position of the balloon during inflation and the combination of a narrow sinotubular junction and the pigtail catheter placed above the balloon were the main reasons for unsuccessful injections. These issues were addressed using a higher pacing rate during BAV and placing the pigtail catheter just above the annulus plane. Of note, it is important to perform the injection once the balloon is completely inflated. At the very beginning of the experience, 4.0 cm, 4.5 cm and 5.0 cm balloon lengths were used. With experience we have learnt that a 4.0 cm balloon is the most appropriate length to obtain good dyeing of the sinuses of Valsalva, as it guarantees enough stability and minimal encumbrance inside the aortic root. Regarding the diameter of the balloon, we decided to use balloons 1-3 mm undersized in relation to the prosthesis which had been recommended to minimise the risk of annular injury during BAV. We found that a BAV catheter 1-3 mm undersized was a good compromise to obtain a satisfactory annulus size estimation and to reduce the risk of annular injury.
IMPACT ON TAVR PROCEDURE
In almost 11% of patients, simultaneous angiography resulted in a change of prosthetic size in relation to the recommendations provided by the non-invasive imaging examinations. More frequently (75%) a larger size was used, because the injection during BAV revealed the presence of contrast dye regurgitation into the left ventricle along the border of the balloon, thus suggesting that the annulus may accommodate a larger size valve more effectively. Interestingly, in 50% of cases in which a change of prosthesis size was adopted, no MSCT assessment of the annulus area was performed, thus further confirming the higher reliability of this strategy compared to TEE or TTE in choosing the correct device size and reducing the incidence of PVR5. The appropriateness of the change of strategy was highlighted by the 100% device success in these patients.
On the other hand, a smaller balloon-expandable valve was used in three cases where angiography during BAV highlighted some cusp calcifications “tenting” toward the walls of the sinuses of Valsalva. This aspect is extremely important, probably more for the balloon-expandable prosthesis, since it expresses a significantly higher radial force to the annulus during the deployment than the self-expanding device. In this series, a lower ES size was chosen in three patients after simultaneous contrast injection and BAV. This strategy probably contributed to preventing potential annular injuries, enhancing the safety of the procedure. In fact, it was recently demonstrated that severe oversizing was associated with contained rupture of the aortic root in balloon-expandable TAVR4. However, one case of annular rupture was reported in the Angio+BAV group, even though it occurred during the very early experience of the technique. Besides the suboptimal dyeing of the sinuses, the limited experience of the technique we had at that time, and the location of a bulky calcification in the right cusp prevented us from appreciating its movement in a left oblique projection. In this case, a right oblique view could help us in visualising the right coronary cusp better. This finding suggests that a learning curve should also be considered in interpreting the images. However, the mechanism of annular rupture after TAVR is not completely understood as yet, and whether this particular technique is able to predict potential annular injury remains unknown.
Furthermore, the contrast injection was useful to ensure the patency of the coronary ostia, which may be occluded by the calcified cusps crushed against the wall of the sinuses of Valsalva. In that case, in order to reduce the risk of this complication, the operator may be able to adopt a few strategies: a) implanting the prosthesis slightly lower into the LVOT in order to reduce the movement of the cusps toward the coronary ostia and the sinus walls; b) placing a wire in the periphery of the left anterior descending, in order to be ready to restore the patency of the coronary ostia by stenting the left main, if it should be needed. In two patients where the aortography during BAV showed the left main ostia threatened by a bulky calcification, a wire was advanced into the left anterior descending before ES implantation.
Limitations
This study has two main limitations. First, the sample size is relatively small, even though it represents the largest series investigating this technique in a systematic fashion. Second, although the balloons were pre-measured, their actual diameter may still have varied from the diameter provided by the manufacturer according to the inflation pressure.
Conclusions
This pilot study showed that, in patients with severe aortic valve stenosis, a technique of simultaneous aortography and balloon valvuloplasty as an adjunct to non-invasive imaging modalities for transcatheter prosthesis selection is feasible, and leads to a change in TAVR strategy in a modest number of patients.
Conflict of interest statement
The authors have no conflicts of interest to declare.

