
Abstract
Aims: The aim of this study was to investigate whether minimising trauma to the aortic annulus and left ventricular outflow tract reduces the occurrence of new conduction disorders and the need for permanent pacemakers.
Methods and results: A total of 175 patients (58% female, mean age 83±6 years) underwent transfemoral TAVI with the Boston Scientific ACURATE neo at three centres in Europe. Prosthesis size selection was based on perimeter-derived annular diameter. Predilatation was performed in all with a balloon 1.9±0.9 mm smaller than the perimeter-derived annular diameter. Post-dilatation was performed in 46 (26.3%) with a balloon 1.2±0.9 mm smaller than the perimeter-derived annular diameter. Eighteen patients (10.3%) developed a new left bundle branch block, 13 (7%) a new first-degree AV block, and four (2.3%) received a new permanent pacemaker. Paravalvular regurgitation was none/trace in 66 (37.7%), mild in 101 (57.7%) and moderate in eight (4.6%). At 30 days, the rate of any stroke was 1.7% (3/175), and one patient (0.6%) had died.
Conclusions: With careful selection of the balloon and the ACURATE neo prosthesis size, very low rates of new conduction disorders and permanent pacemaker implantation may be achieved without increasing the amount of paravalvular regurgitation.
Abbreviations
AVB: atrioventricular block
BBB: bundle branch block
LBBB: left bundle branch block
PAR: paravalvular regurgitation
RBBB: right bundle branch block
TAVI: transcatheter aortic valve implantation
THV: transcatheter heart valve
Introduction
Transcatheter aortic valve implantation (TAVI) has been successfully performed in inoperable, high-risk, and intermediate-risk patients with low mortality and complication rates1,2. Next-generation valves have been designed to improve annular sealing and reduce paravalvular regurgitation, but it appears that this comes at the price of an increased rate of new conduction disorders3,4. In particular, the occurrence of a new left bundle branch block and the need for a permanent pacemaker remain a matter of concern5,6.
The self-expanding ACURATE neo™ TF system (Boston Scientific, Marlborough, MA, USA) received CE-mark approval in 2014 and has design features aimed at preventing new conduction disorders. It has been associated with low pacemaker rates in the TF89 cohort and the recently presented SAVI TF 1,000-patient registry7.
Aside from device development, optimising the procedure and establishing best practices may further reduce complication rates8. In a recent study using the CoreValve® prosthesis (Medtronic, Minneapolis, MN, USA), predilatation with a smaller valvuloplasty balloon resulted in significantly lower pacemaker implantation rates9.
In our series, we aimed to assess whether the rate of conduction disorders and pacemaker implantations after ACURATE neo implantation can be further reduced by careful selection of the balloon size of predilatation and post-dilatation balloons, hence minimising the trauma to the aortic annulus and left ventricular outflow tract.
Methods
STUDY POPULATION
All patients undergoing TAVI for the treatment of severe aortic stenosis with the self-expanding ACURATE neo transcatheter heart valve at three centres in Europe, the Heart Center Lucerne (Switzerland, n=78), the Karolinska University Hospital (Stockholm, Sweden, n=66), and the Odense University Hospital (Odense, Denmark, n=31), were analysed. Data were collected throughout the initial hospital stay and follow-up was conducted at 30 days post procedure. The study complies with the Declaration of Helsinki. Prospective data acquisition after TAVI was approved by all ethics committees. All patients provided written informed consent for the TAVI procedure and for prospective data acquisition and follow-up examinations.
TAVI WORK-UP, PROCEDURE, AND POST-PROCEDURAL MONITORING
Potential TAVI candidates were discussed by the interdisciplinary Heart Team consisting of non-invasive cardiologists, interventional cardiologists, and cardiac surgeons. The valve size was chosen according to the perimeter of the annulus (a small “S” valve for annular perimeters <72 mm, a medium “M” valve for annular perimeters between 72 and 78 mm, and a large “L” valve if the perimeter was between 79 and 84 mm). In borderline cases, the larger valve was preferred. Due to the relatively low radial force of the inflow portion of the ACURATE neo THV, predilatation with a valvuloplasty balloon was performed in all patients (Figure 1). To minimise trauma to the annulus and the underlying conduction system, the diameter of the balloon was chosen 1-3 mm smaller than the perimeter-derived annular diameter. Post-dilatation was only performed in case of relevant aortic regurgitation or relevant antegrade flow gradient (>10 mmHg) with a balloon 1-2 mm smaller than the perimeter-derived annular diameter. Following TAVI, patients were monitored for one to three days, depending on the presence of a bundle branch block (BBB) or an atrioventricular block (AVB). In addition, an ECG was performed prior to discharge. The decision to implant a permanent pacemaker was left to the discretion of the operator.
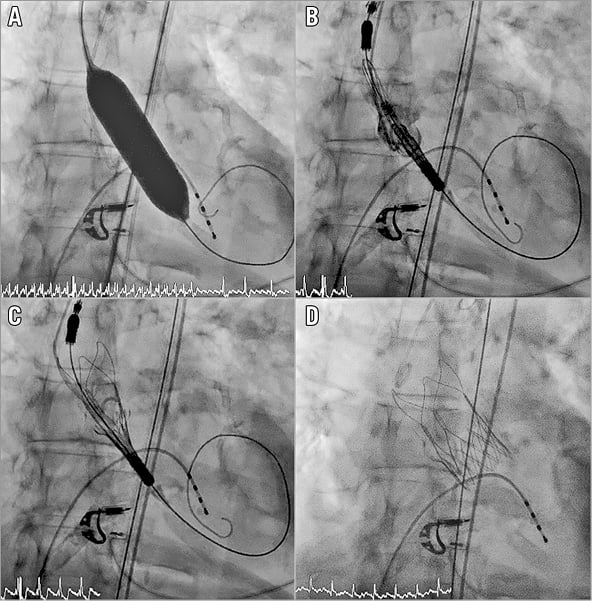
Figure 1. Implantation of the ACURATE neo transcatheter heart valve. Predilatation with a valvuloplasty balloon is generally recommended due to the relatively low radial force of the inflow part of the valve prosthesis (A). The valve is positioned (B) and released top down (C & D).
DEFINITIONS
Clinical endpoints were defined according to the updated definitions of the Valve Academic Research Consortium (VARC)10. Conduction disorders included bundle branch blocks and atrioventricular blocks. A first-degree AVB was defined as a PQ time ≥200 milliseconds (ms). A complete BBB was defined as a QRS duration ≥120 ms and the typical pattern of either left bundle branch block (LBBB) or right bundle branch block (RBBB). QRS duration <120 ms was classified as no BBB. Implantation depth was measured on post-procedural supravalvular angiography as the distance between the lowest part of the THV frame and the ventricular tip of the non-coronary cusp. Valve oversizing was calculated as the difference between the nominal diameter of the THV (23, 25, and 27 mm for the S, M, and L THV, respectively) and the perimeter-derived annular diameter. Balloon undersizing was calculated as the difference between the perimeter-derived annular diameter and the diameter of the valvuloplasty balloon.
STATISTICAL ANALYSIS
If not otherwise indicated, data are presented as mean±standard deviation for continuous variables and as number and frequency for categorical variables. Continuous parametric variables were compared using the Student’s t-test. Categorical variables were compared using the chi-square test or Fisher’s exact test as appropriate. A logistic regression analysis was performed to calculate the odds ratio (OR) and the 95% confidence interval (CI) of variables predicting new conduction disorders at a significance level of p<0.05. Statistical analyses were conducted with Stata version 13 (StataCorp, College Station, TX, USA) and tested using two-sided tests at a significance level of 0.05.
Results
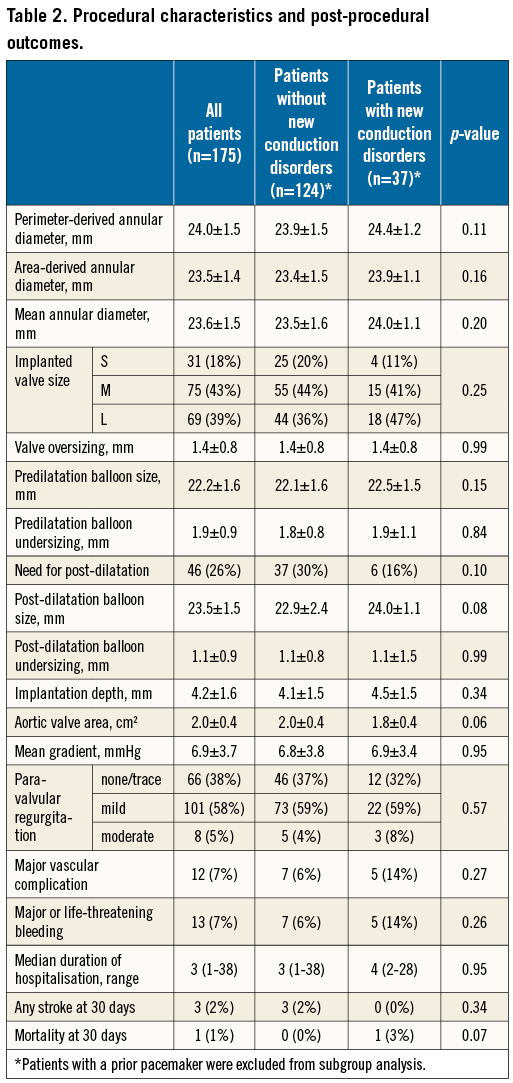
From June 2015 to May 2016, 175 patients (58% female) with a mean age of 83±6 years were enrolled. Fourteen patients (8.0%) had a permanent pacemaker at baseline. No “prophylactic” pacemaker implantations were performed before TAVI (e.g., pacemaker implantation in a patient with pre-existing RBBB and concomitant first-degree AV block). Baseline characteristics are summarised in Table 1.
TAVI PROCEDURE AND OUTCOMES
Procedural characteristics and outcomes are listed in Table 2. All patients underwent transfemoral TAVI with the ACURATE neo THV. Valve sizing was performed via ECG-gated computed tomography (n=166, 95%); in patients with severe chronic kidney disease, magnetic resonance tomography (MRT) without the administration of gadolinium was carried out instead (n=9, 5%). The procedure was performed under local anaesthesia with mild conscious sedation in all patients. Predilatation was performed in all with a balloon diameter 1.9±0.9 mm smaller than the perimeter-derived annular diameter. Post-dilatation was performed in 46 patients (26.3%) with a balloon diameter 1.1±0.9 mm smaller than the perimeter-derived annular diameter. TAVI reduced the mean transaortic gradient from 47±15 mmHg to 7±4 mmHg (p<0.01) and increased the aortic valve area from 0.7±0.2 cm2 to 2.0±0.4 cm2 (p<0.01). Paravalvular regurgitation (PAR) was none/trace in 66 (37.7%), mild in 101 (57.7%) and moderate in 8 (4.6%). Patients were discharged after a median of three days post procedure (range 1-38 days). The 30-day stroke rate was 1.7% (3/175), and one patient (0.6%) died (Table 3). This patient died from coronary obstruction after the first THV was implanted too high and implantation of a second THV was required (SAPIEN 3; Edwards Lifesciences, Irvine, CA, USA).
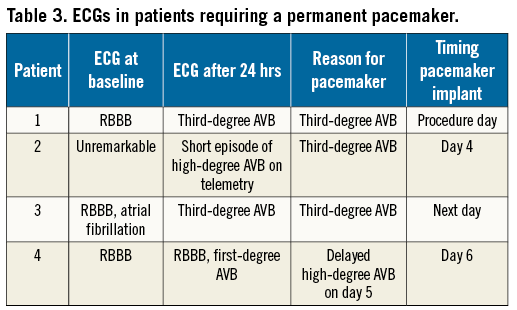
NEED FOR A NEW PERMANENT PACEMAKER AND CONDUCTION DISORDERS
At discharge, 18 (10.3%) patients had a new LBBB, one (0.6%) a new RBBB, and 11 (6.3%) a new first-degree AVB (Figure 2). Implantation of a new permanent pacemaker was required in four patients (2.3%, excluding patients with a prior pacemaker: 2.5%). Of these, three (75%) had a complete RBBB at baseline (OR 39.8, 95% CI: 3.8-411.8, p<0.01). Significant predictors for new conduction disorders were the presence of diabetes (OR 2.8, 95% CI: 1.2-6.4, p=0.01), beta-blocker at baseline (OR 2.2, 95% CI: 1.0-4.6, p=0.046), and a lower mean transaortic gradient at baseline (OR 0.97, 95% CI: 0.94-0.99, p=0.046).
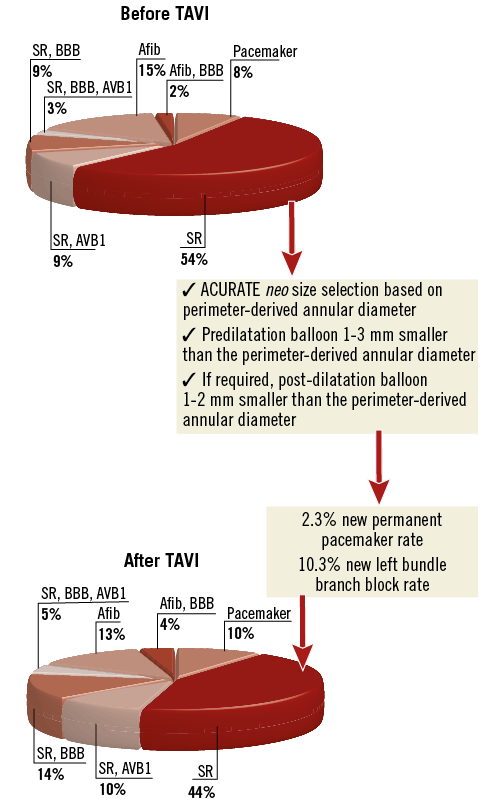
Figure 2. ECG findings at baseline and before discharge. With careful selection of the valvuloplasty balloon and the transcatheter heart valve size, a new left bundle branch block occurred in 17 (10.0%) patients, and only four (2.3%) required implantation of a new permanent pacemaker.
Discussion
To the best of our knowledge, this study reports the lowest pacemaker rate that has ever been published after TAVI in a large series of patients. As TAVI relies on oversizing to anchor the device within the native annulus and thus applies force to adjacent structures such as the conduction system, many experts believe that pacemaker rates after TAVI will always be higher than after open heart surgery. In particular, self-expanding valves have been associated with high pacemaker rates, ranging between 10 and 35%11-14. Our study, however, shows that, with careful selection of the balloon and prosthesis size, it is possible to achieve a very low pacemaker rate that compares well to surgical aortic valve implantation with new permanent pacemaker ranging between 3.6 and 7.1% in recent trials15-17. The pacemaker rate was only 2.3% including patients with a prior pacemaker and 2.5% after excluding such patients. Similarly, the rate of new LBBB was only 10%. Furthermore, 30-day mortality was very low with only one patient who died (0.6%). Notably, these outcomes did not come at the price of increased moderate PAR which was still below 5%. However, the rate of mild PAR was relatively high in this study (58%), and only 38% of patients had none/trace PAR. The next generation of the ACURATE neo, the ACURATE neo AS, is currently being studied in a CE-mark trial. This THV will feature a modified skirt aimed at reducing the rate of PAR. Whether this modification will result in higher rates of new LBBB or new permanent pacemakers is not yet known.
PATHOPHYSIOLOGY OF NEW CONDUCTION DISORDERS
New conduction disorders after TAVI represent frequent complications, particularly after implantation of self-expanding THVs2,17. Such valves apply a continuous radial force to the annular tissue and, in case of a deeper implantation, also to the left ventricular outflow tract. The target implantation depth of the ACURATE neo valve is about 4 mm below the annular level. The very low rate of LBBB and the need for a permanent pacemaker observed in the present study might appear surprising at first. However, the radial force of the ACURATE neo THV is relatively low, especially at the inflow portion of the frame, which may be in the immediate vicinity of the AV conduction system (Figure 3). This distribution of radial force may explain our study results. Previous studies have identified several risk factors for implantation of a permanent pacemaker, including pre-existing conduction disorders such as RBBB, bradycardia and the presence of AV block, membranous septum height, cusp calcification, implantation of a self-expanding valve, implantation depth, and post-procedural conduction disorders18,19. However, there may be differences among different THVs. In fact, in our series, there was no difference in implantation depth or the degree of valve oversizing between patients who experienced a new conduction disorder and those who did not. Furthermore, the ACURATE neo THVs are available in 2 mm increments, which may allow more precise sizing.
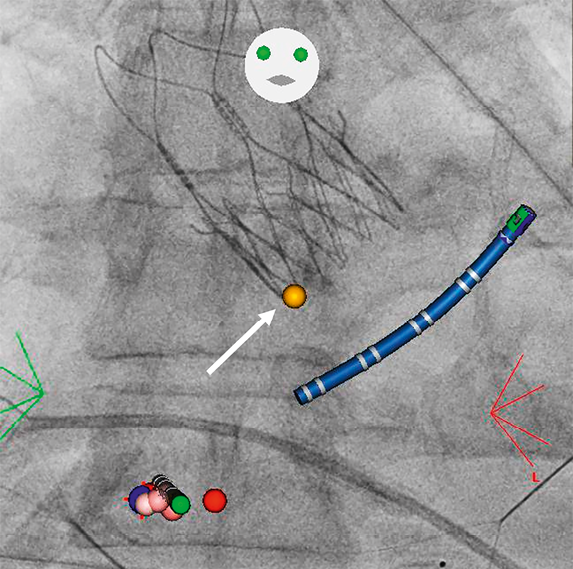
Figure 3. Prosthesis location in relation to the atrioventricular node. This image was taken in a patient who underwent radiofrequency ablation for atrial flutter three months after implantation of an ACURATE neo transcatheter heart valve using the CARTO® 3 CARTOUNIVU™ System (Biosense Webster, Irvine, CA, USA). The yellow dot (arrow) shows the location of the atrioventricular node, in the immediate vicinity of the inflow portion of the valve frame. Red and pink points show the ablation line; the catheter is shown in blue.
Van Gils et al compared the rate of permanent pacemaker implants in patients with baseline RBBB treated with different THVs. As baseline RBBB is a known predictor for pacemaker implants, rates were high at 75% for LOTUS™ (Boston Scientific), 46% for CoreValve, 32% for SAPIEN XT (Edwards Lifesciences) and 34% for SAPIEN 320. With the caveat that only 15 patients with a baseline RBBB were present in our series, only 20% of patients with RBBB (3/15) received a pacemaker. Furthermore, three out of four patients with a pacemaker implant had an RBBB at baseline.
RELEVANCE OF NEW CONDUCTION DISORDERS
Available data on the clinical relevance of conduction disorders after TAVI remain controversial21. The presence of a new LBBB has been associated with an increased risk of cardiac death21,22 and a lack of improvement in ejection fraction23. The need for a new permanent pacemaker has been associated with reduced survival in some studies24, but not all21,25,26. A recent meta-analysis has even found a trend towards a protective effect from cardiac death in the first year after the procedure21. One possible explanation may be that patients with new-onset LBBB are at higher risk for cardiac death because of the potential progression towards complete AVB and sudden death, which possibly may have been prevented by implantation of a permanent pacemaker21,27. Furthermore, evidence indicates that a subset of these conduction disorders may resolve over time, and not all patients receiving a permanent pacemaker may actually be paced during follow-up28,29. Not surprisingly, new conduction disorders and implantation of a permanent pacemaker have been linked with prolonged hospital stay and costs30. New conduction disorders complicate in-hospital management, as patients with a new LBBB or a new first-degree AVB may progress towards complete AVB and therefore require telemetry monitoring. A recent publication suggested that such patients should be monitored until the ECG remains stable for at least 48 hours. On the other hand, the risk of delayed high-degree AVB was extremely low in the absence of relevant conduction disorders after TAVI. In the absence of other complications, patients without relevant conduction disorders after TAVI may be candidates for safe, early discharge31. In summary, a low rate of conduction disorders, particularly LBBB and first-degree AVB, and a low rate of new permanent pacemakers may facilitate in-hospital patient management, reduce the duration of in-hospital stay, and costs, and might improve long-term prognosis.
Study limitations
This is a retrospective analysis of prospectively acquired data and is therefore subject to the limitations of such a study design. The patient number is relatively small. No core laboratory analysis was performed, but ECGs and echocardiograms were analysed by experienced cardiologists. Further, calcification was not systematically assessed. Due to the low number of patients with new conduction disorders, no multivariable logistic regression analysis was performed.
Conclusions
With careful selection of the valvuloplasty balloon and the ACURATE neo prosthesis size, very low rates of new conduction disorders and permanent pacemaker implantation may be achieved without increasing the amount of paravalvular regurgitation. This may facilitate post-procedural patient management, reduce the duration of in-hospital stay, and costs, and may ultimately improve long-term prognosis.
| Impact on daily practice Predilatation, or (if applicable) post-dilatation, choosing a balloon size 1-2 mm smaller than the perimeter-derived annular diameter, was associated with very low conduction disorders and permanent pacemaker rates following transfemoral implantation of the ACURATE neo transcatheter heart valve. With this technique, it appears that the permanent pacemaker rate may be reduced below the level of surgical aortic valve implantation. This may lead to a paradigm shift such that TAVI does not essentially have to be associated with higher rates of permanent pacemakers. |
Conflict of interest statement
S. Toggweiler serves as a proctor and consultant to Symetis/Boston Scientific, is a consultant to NVT, and has received speaker fees from Symetis/Boston Scientific, Edwards Lifesciences and Medtronic. A. Rück serves as a proctor to Symetis/Boston Scientific and has received grants from Medtronic, St. Jude Medical and Symetis/Boston Scientific. The other authors have no conflicts of interest to declare.

