Abstract
Aims: We investigated the impact of the diameter of the valvuloplasty balloon (VB) used for predilation before transcatheter aortic valve implantation (TAVI) on atrioventricular block formation with consecutive need for permanent pacemaker (PP) implantation.
Methods and results: TAVI was performed in 269 consecutive patients using the CoreValve prosthesis (Medtronic) via transfemoral access under local anaesthesia with mild analgesic medication. After exclusion of 32 patients with previously implanted PP, 237 patients were included in a retrospective analysis of the impact of VB size on subsequent PP incidence. Implantation success rate was 99.3%. Periprocedural mortality was 0%, and 30-day mortality was 5.9%. PP implantation after TAVI was required by 21.1%. Of 114 patients treated by 25 mm balloon valvuloplasty, a PP was implanted in 27.1%. In 123 patients, who were treated by VB with a ≤23 mm diameter, the PP implantation rate decreased to 15.4% (p=0.04). In univariate analysis, larger VB size resulted in a greater prevalence of PP implantation after TAVI. After adjustment by multivariate analysis for baseline clinical and operative characteristics, VB size remained an independent predictor of PP implantation.
Conclusions: Moderate balloon predilation in patients undergoing TAVI with the Medtronic CoreValve prosthesis reduces the PP rate without affecting procedural success.
Abbreviations
LBBB: left bundle branch block
RBBB: right bundle branch block
PP: permanent pacemaker
SAVR: surgical aortic valve replacement
TAVI: transcatheter aortic valve implantation
VB: valvuloplasty balloon
Introduction
Following the onset of clinical symptoms, severe aortic stenosis has a poor prognosis with conservative treatment, and is associated with a high mortality rate1-3. Surgical valve replacement has emerged as a therapy of choice for aortic stenosis over recent decades, dramatically improving symptoms and survival4,5. However, surgical valve replacement in elderly patients with important comorbidities may result in severe complications and increased postoperative mortality. Thus, up to 30% of patients suffering from severe symptomatic aortic stenosis at present are not receiving surgical valve replacement6.
Recently, an alternative, endoluminal approach to aortic valve replacement emerged. Since its introduction in 20027, transcatheter aortic valve implantation (TAVI) has emerged as an alternative treatment strategy for elderly patients with severe aortic valve stenosis considered to be at high risk for surgical treatment. Even though TAVI can be performed in selected patients with outcomes comparable to surgical valve replacement (SAVR)8-10, serious complications such as stroke, paravalvular regurgitation, coronary artery occlusion or conduction disorders necessitating pacemaker implantation can occur.
Following left bundle branch block (LBBB), atrioventricular block is the second most common acquired conduction disorder following TAVI, with a reported incidence of 16%-42.5%11,12. As opposed to a mere LBBB, atrioventricular block requires immediate pacemaker activity and subsequent PP implantation. Current evidence suggests that the PP implantation rate due to complete atrioventricular block is more frequent after TAVI of CoreValve prostheses (Medtronic Inc., Minneapolis, MN, USA) compared to SAPIEN XT prostheses (Edwards Lifesciences LLC, Irvine, CA, USA)9,12,13.
During TAVI procedures involving Medtronic CoreValve devices, we noticed that atrioventricular block occurred more frequently after utilisation of a larger VB. Therefore, we sought to investigate the impact of the valvuloplasty balloon size on the incidence of a complete atrioventricular block, subsequently requiring a PP implantation, after Medtronic CoreValve implantation.
Methods
PATIENTS
Between November 2007 and August 2011, 269 (124 male, mean age 82 yrs [range: 53-99]) consecutive patients with symptomatic severe aortic valve stenosis (New York Heart Association [NYHA] Class ≥II) and one patient with aortic regurgitation grade IV and no surgical option underwent TAVI (Medtronic CoreValve) in our centre. Patient screening routinely included transthoracic echocardiography, multislice CT and coronary angiography.
Before TAVI was performed, all patients were discussed in our aortic valve multidisciplinary team meeting. Adequate arterial access and aortic annulus diameter suiting either the 26 mm or the 29 mm CoreValve were prerequisites for TAVI. Patients with high-grade coronary artery stenosis underwent PCI before TAVI. Severe renal insufficiency and mitral regurgitation up to grade III (0-IV) were not considered as exclusion criteria. Written informed consent was obtained from all patients before undergoing TAVI. Further patient characteristics are shown in Table 1.

DEVICE DESCRIPTION AND IMPLANTATION PROCEDURE
The third-generation 18 Fr device of the self-expanding CoreValve aortic valve prosthesis was used in our series. A more detailed description has been previously reported14,15.
Implantation was done under local anaesthesia with mild analgesic medication by experienced operators (Figure 1). Vascular access was obtained across the common femoral artery using a commercially available percutaneous closure system (Prostar XL structure device; Abbott Vascular, Santa Clara, CA, USA).
Figure 1. Medtronic CoreValve implantation.
PRETREATMENT CT SCAN
Dual source CT scan (Definition Flash; Siemens Medical Solutions, Forchheim, Germany) was performed routinely before TAVI. Aortic annulus diameters as well as the degree of calcification of the aortic valve leaflets and the distance of the coronary arteries from the aortic annulus were measured on a dedicated workstation.
STATISTICAL ANALYSIS
Continuous variables are expressed as mean±SD and were compared using the Student’s t-test. Categorical variables are expressed as numbers and percentages and were compared by using the Fisher’s exact test or χ² test as appropriate. Due to the problem of small event numbers, for modelling the dichotomous outcome “SM post-TAVI”, we first show the univariate analyses. Then we chose five candidate confounders and performed a backward elimination. The level for all confounders was set to 0.1. Overall, 211 complete cases were available. For both models, odds ratios (OR), 95% confidence intervals and p-values resulting from the Wald test are reported. All statistical tests were two-sided and a p-value of <0.05 was considered to be statistically significant. Statistical analyses were performed using the SPSS software package, version 18.0 (SPSS Inc., Chicago, IL, USA), and R software, version 2.13.2.
Results
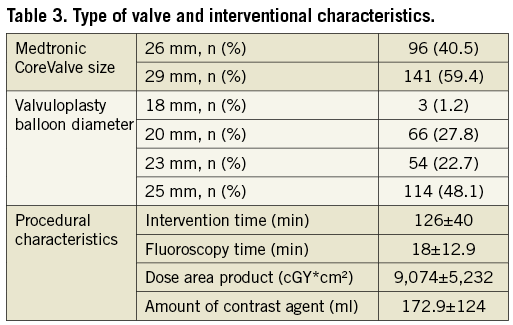
From the initial cohort of 269 patients, 32 had to be excluded because of a previous PP implantation. Of the remaining 237 patients, 123 received the 26 mm prosthesis (VB size: 18/20 mm in 62 patients, 23 mm in 14 patients, 25 mm in 20 patients), whereas 114 received the 29 mm prosthesis (VB size: 18/20 mm in 7 patients, 23 mm in 40 patients, 25 mm in 94 patients). Mean intervention time from arterial puncture until percutaneous closure was 126±40 min, with a mean fluoroscopy time of 18±13 min and a mean contrast agent use of 173±124 ml. Mean stay at the intensive care unit was 3.2±3.3 days, and mean in-hospital stay was 17±9 days.
Successful device implantation was achieved in 236 of 237 patients.
Endpoints regarding in-hospital outcome were defined according to VARC (Valve Academic Research Consortium) criteria. The combined 30-day safety endpoint was defined according to the recommendations as the composite of all-cause mortality, major stroke, life-threatening or disabling bleeding, acute kidney injury (>stage 3) including renal replacement therapy, periprocedural myocardial infarction, major vascular complication and repeat procedure for valve-related dysfunction16 (Table 2 and Table 3).
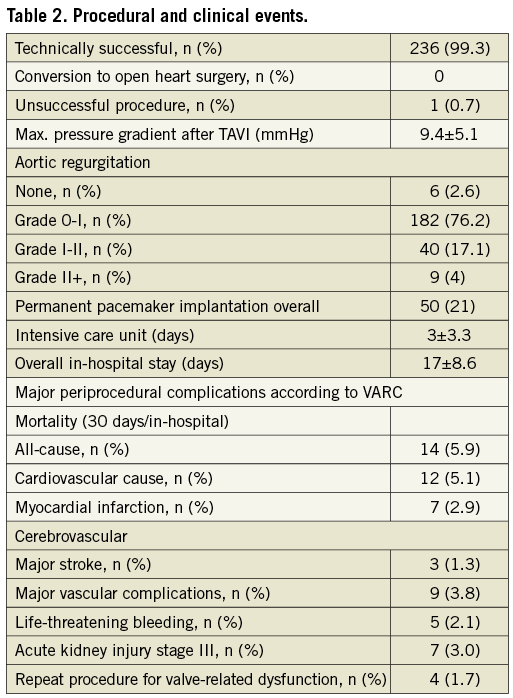
PERMANENT PACEMAKER IMPLANTATION AND PREDILATION
LBBB was the most frequent procedure-related conduction disorder, appearing in 101 patients (42%). Overall, a PP had to be implanted in 21.1% (50/237) of patients after TAVI. Of the de novo implanted permanent pacemakers, 26 PP (18.5%) were required after a 29 mm prosthesis implantation and 24 PP (25%) in patients with a 26 mm prosthesis size. The PP incidence in the 114 patients in whom VB with diameters of 25 mm were used was 27.1% (31 PP). A drop of PP incidence to 15.4% (19) (p=0.042) was observed in the 123 patients in whom only 23 mm or smaller VB diameters were used (Figure 2). Except for one-vessel CAD, prior stent implantation and prior bypass graft surgery, no significant difference in patient characteristics between these two groups was found (Table 1).
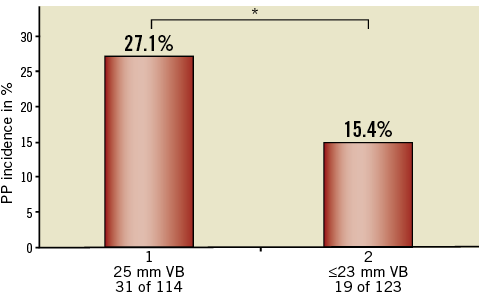
Figure 2. PP rate depending on VB size used. 31 of 114 patients in the 25 mm balloon group needed a PP (27.1%) whereas only 19 of 123 patients predilated with a 23 mm balloon or smaller (15.4%) needed a PP (p=0.04). * indicates a significant difference.
Analysing the PP rates according to the CoreValve prosthesis size and the VB used, a further marked difference was found. In the 26 mm prosthesis subgroup (including patients with aortic annulus sizes from 20-23 mm) a PP rate of 40% (8 of 20) was registered for those patients predilated with a 25 mm VB, decreasing to a PP rate of 28.5% (4 of 14) for patients predilated with a 23 mm VB, and 19.3% (12 of 62) for patients predilated with a 20 mm or 18 mm VB (p=0.083). The seven patients who were predilated with an 18 mm VB had no need for PP implantation (Figure 3A).
In the 29 mm prosthesis subgroup (including patients with aortic annulus sizes from 24-27 mm) a PP rate of 24.4% (23 of 94) was registered for those patients predilated with a 25 mm VB, decreasing to a PP rate of 7.5% (3 of 40) for patients predilated with a 23 mm VB (p=0.033), and 0% for patients predilated with a 20 mm or an 18 mm VB (Figure 3B).
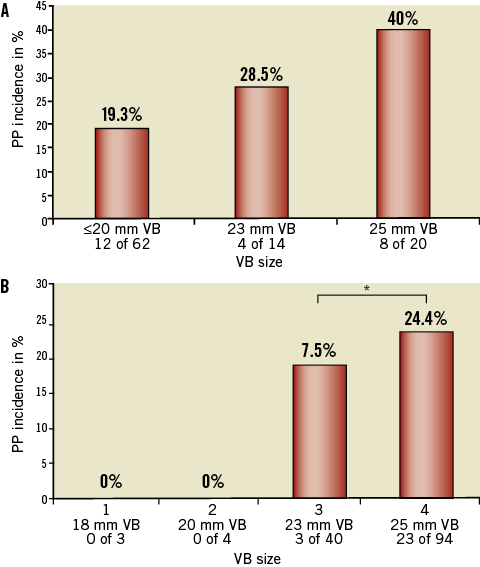
Figure 3. PP rates in patients with 26 mm or 29 mm prosthesis size depending on VB size used. A) A marked reduction of PP rates was found in patients with a 26 mm prosthesis size depending on the VB size used for predilatation. A 40% PP rate in patients predilatated with a 25 mm VB balloon and a 19.3% PP rate in patients predilatated with a 20 mm or 18 mm VB balloon (p=0.08). B) Also, in patients with a 29 mm prosthesis size, PP rates depend on the VB size used for predilatation (*p<0.05).
On univariate analysis for baseline clinical and operative characteristics (including pre-existing LBBB, pre-existing RBBB, pre-existing AV block I°, interventricular septal diameter, valve height position and balloon/annulus ratio), VB size was a predictor of PP implantation with an odds ratio (OR) of 2.31 (95% CI: 1.11-4.82, p=0.025) (Table 4).

On multivariate analysis, where, apart from balloon size, pre-existing right bundle branch block (RBBB) and pre-existing AV block I° were selected as confounders, a pre-existing RBBB with an OR of 46.71 (95% CI: 8.76-249.03, p≤0.001) and VB of 25 mm with an OR of 5.45 (95% CI: 1.02-28.99, p=0.046) were the only independent predictors of PP implantation. The OR for PP using a balloon size of 23 mm compared to a ≤20 mm balloon was 1.02 (95% CI: 0.13-8.02, p=0.98). The OR for PP with pre-existing AVB I° was 0.24 (95% CI: 0.04-1.57, p=0.13) (Table 5, Figure 4).
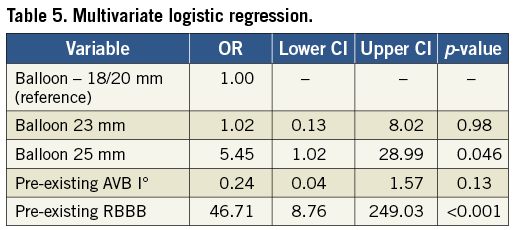
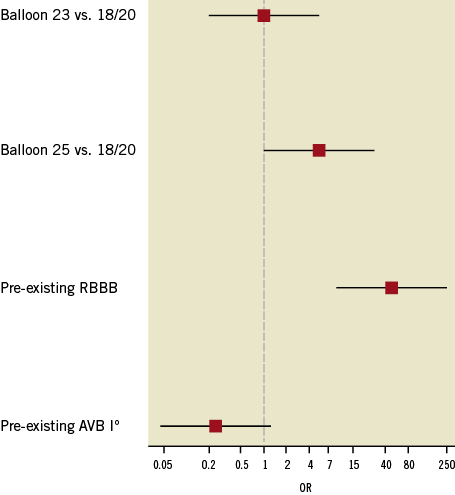
Figure 4. Odds ratio (OR) for pacemaker (PP) implantation. When compared to smaller balloons (18/20 mm), the 23 mm balloon did not increase PP implantation frequency. However, the 25 mm balloon and a pre-existing right bundle branch block (RBBB) induced a significantly higher OR (p=0.041 and p<0.001, respectively). In contrast, a pre-existing AV block (AVB) I° did not significantly affect PP implantation frequencies.
Discussion
Atrioventricular blocks of second or third degree with symptomatic bradycardia are a known complication of surgical aortic valve replacement as well as after TAVI. Although this side effect is routinely treated by PP implantation, severe complications can occur. Translocation of the temporary pacemaker lead in patients with complete atrioventricular block can trigger a potential life-threatening bradycardia or cardiac arrest. Moreover, atrial and ventricular perforation triggering pericardial tamponade during PP implantation may occur in an emergency. Thus, reduction of PP incidence after TAVI is a most appropriate goal.
During the TAVI procedure, valvuloplasty may cause oedema, inflammation, mechanical trauma or even localised haematoma affecting the tissue surrounding the aortic bulbus and left ventricular outflow tract. This area is localised in close proximity to the AV node on the right side of the interventricular septum near the left coronary cusp and the left bundle branch. Moreno et al17 reported a case of compression of the bundle of His by a localised haematoma at the interventricular septum found during necropsy in a patient with complete atrioventricular block following TAVI.
Fraccaro et al18 found the depth of prosthesis implantation and pre-existing RBBB to be independent predictors of PP implantation after CoreValve implantation in a cohort of 70 consecutive patients. The lower one third of the CoreValve prosthesis is characterised by high radial forces to secure safe anchoring. Therefore, it is likely that a deeper implantation site of the prosthesis is associated with a greater risk of conduction bundle compression and, consequently, development of severe conduction disorders. Though in that study VB diameter was not a predictor for PP implantation18, VB sizes were not included, potentially differing from the VB sizes used in our study where implantation depth was not a significant predictor for subsequent PP implantation. In addition, this difference might be explained by the fact that from the beginning we tried to choose a high implantation plane.
Other clinical studies revealed further predictors for PP implantation such as large size of the prosthesis19, or small diameter of the left ventricular outflow tract20. The diameter of the aortic annulus also seems to be of relevance for conduction disorders after the CoreValve procedure21. These data suggest that the anatomic dimensions or the size of local trauma induced could play in important role in the pathophysiology of post-procedure conduction disorders.
In our series, a valvuloplasty balloon of greater diameter was found to be an independent predictor of PP implantation. It is conceivable that a larger valvuloplasty balloon causes more damage to the conduction system than a balloon of smaller diameter, thus increasing the probability of permanent impairment of the conduction system. However, if performed alone, valvuloplasty generates a mere 1% rate of permanent pacemaker implantation in a current setting22. Obviously, the valvuloplasty-associated PP rate is altered by a subsequent TAVI procedure. Therefore, we propose a two-hit model, in which the trauma inflicted by a larger valvuloplasty balloon is a first hit usually insufficient to generate a persistent and complete atrioventricular block, unless a second hit is applied to the conduction system by the valve frame. Again, the greater the diameter of the frame, the worse is the impact on the conduction system (Figure 3A, Figure 3B). The multivariate analysis revealed VB size to be an independent risk factor for incidence of PP. In our collective, lower PP incidence occurred after ≤23 mm VB use than after 25 mm VB use.
Consistently, Nuis et al demonstrated that new conduction abnormalities in CoreValve procedures occurred in half of the cases before the final valve implantation. These findings support our results that VB size seems to be of relevance for the future need of PP after TAVI21. It is worth noting that post-dilation VB size apparently does not exert the same impact23, indicating that valvuloplasty of an implanted valve stent is different from an unprotected balloon valvuloplasty.
Several reports have indicated higher PP implantation rates in CoreValve prostheses than after SAPIEN implantation20,24-26. The shorter design of the SAPIEN valve most likely avoids compression of the left ventricular outflow tract and adjacent conduction system. Noteworthy, a 20 mm VB is used for predilation for the 23 mm SAPIEN valve and a 23 mm VB for the 26 mm SAPIEN valve. These smaller VB diameters could have an additional impact on lower PP rates.
PP rates reported after implantation of CoreValve prostheses vary between 16% and 42%11,12. Varying PP rates are partially explained by hospital-specific PP implantation criteria. Here, PP implantation was triggered by AV block III° at any time point during or after TAVI, or by symptomatic bradycardia after TAVI. An asymptomatic LBBB, however, was not viewed as a PP indication. Asymptomatic AV blocks of I° and II° or asymptomatic trifascicular blocks were not indications for PP implantation. No prophylactic PP implantations were performed. We are well aware of different policies (at times including electrophysiological studies after TAVI) and possible recovery of conduction defects within 10 days. However, during a postprocedural observation time of 3.2 days (ICU) and 11.2 days (total), we did not notice symptomatic bradycardias. After discharge, however, two sudden cardiac deaths occurred in our series, potentially triggered by symptomatic bradycardia, although a ventricular tachycardia after myocardial infarction has not been ruled out.
Another concern regarding moderate predilation is impaired self-expansion due to residual aortic stenosis, resulting in higher-grade aortic regurgitation after the procedure. We did not find any differences in aortic regurgitation grades among patient groups with different predilatation strategies. Consistently, Grube et al23 found no difference in aortic regurgitation with or without balloon predilation in 60 CoreValve implantations. The reported PP rate of 11.7% is similar to the 14.1% found in our cohort after disregarding 25 mm balloons for predilation.
Limitations
First, the major limitation of our study is its single-centre retrospective non-randomised design. Second, we cannot completely exclude symptomatic bradycardia after patient discharge and recovery of conduction defects after PP implantation. However, criteria for PP implantation did not change over the inclusion period. Third, while pre-existing RBBB was significantly associated with the need for PPI after CoreValve implantation, the exact determination of the associated risk (point estimate of OR) warrants further investigation in larger cohorts due to the low prevalence of pre-existing RBBB observed here.
Conclusion
To conclude, our analysis suggests that a balloon size below 25 mm is capable of reducing the need for permanent pacemaker implantation after Medtronic CoreValve-based TAVI. Although the choice of a smaller valve is associated with a reduced need for PP implantation, this choice is made based on morphologic criteria of the outflow tract and aortic bulbus, and cannot usually be based on the consideration of PP requirement.
| Impact on daily practice The need for pacemaker implantation after the CoreValve procedure is still a frequent complication.Therefore, in daily practice the use of smaller VB could be an easy and safe tool to reduce PP rates after CoreValve implantation. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

