
Abstract
Aims: The aims of the study were to evaluate balloon post-dilation (BPD) and valve-in-valve (ViV) implantation for the reduction of paravalvular leakage (PVL) in patients undergoing transcatheter aortic valve implantation (TAVI) with use of the self-expanding CoreValve prosthesis and to assess whether the aortic regurgitation (AR) index can be used to quantify the reduction of PVL by these corrective measures.
Methods and results: Angiography and the AR index were used to evaluate the severity of PVL before and after corrective measures in patients suffering from more than mild PVL. Corrective measures were performed in 44.7% (101/226 patients): BPD was performed in 85 patients and ViV implantation in 16 patients, respectively. In 86% (87/101 patients), PVL reduction was successful (no or mild PVL). BPD increased the AR index from 19.1±11.0 to 25.9±5.8 (p<0.001) and ViV implantation from 17.6±6.4 to 29.5±9.1 (p=0.008). One-year mortality (21.6% vs. 17.6% vs. 25.0%; p=0.69) and procedural stroke rate (2.4% vs. 2.4% vs. 0%; p=0.82) were not different between patients without corrective measures compared to patients who had undergone corrective measures (BPD or ViV).
Conclusions: BPD and ViV implantation are safe and effective to reduce PVL in TAVI patients. The AR index is useful to quantify the success of these corrective measures for PVL reduction objectively.
Introduction
Transcatheter aortic valve implantation (TAVI) has become established as an alternative treatment option for patients suffering from severe aortic stenosis with high risk or contraindication for surgical aortic valve replacement (SAVR)1,2. However, the occurrence of paravalvular leakage (PVL) caused by incomplete circumferential apposition of the prosthesis stent frame at the level of the aortic annulus due to calcifications, suboptimal placement of the prosthesis, and/or annulus-prosthesis size mismatch is a procedure-related shortcoming of TAVI3-10.
PVL has been documented in up to 70% of all patients undergoing TAVI11-14, whereas more than mild PVL has been reported in approximately 10-15% of all TAVI patients1,15-19. As significant PVL has a detrimental impact on short- and long-term mortality11,13-16,20-24, a multimodal approach combining invasive haemodynamic measurements with determination of the aortic regurgitation (AR) index and non-invasive imaging modalities such as angiography and echocardiography seem to be useful to quantify the degree of PVL in the acute implantation setting immediately after deployment of the transcatheter heart valve (THV), and can be used to identify patients who might benefit from corrective measures. However, imaging modalities are limited due to, for example, patient position in the cathlab, constraints on contrast dye use, and operator dependency. Objective, simple and reproducible quantification is needed in these situations but is frequently not easy to accomplish.
Balloon post-dilation (BPD) can reduce PVL by achieving a better expansion of the prosthesis stent frame and an optimal sealing of the paravalvular space. Valve-in-valve (ViV) implantation is another option to overcome significant PVL, especially if the implantation height turns out to be suboptimal13,25-30.
The aim of our study was to evaluate whether BPD and ViV implantation are effective and safe in patients undergoing TAVI with use of the self-expanding CoreValve® prosthesis (Medtronic, Minneapolis, MN, USA) and to assess whether the AR index can be used to quantify the reduction of PVL by these corrective measures.
Methods
PATIENT POPULATION AND TAVI PROCEDURES
From June 2011 to December 2013, 226 consecutive patients underwent TAVI using the self-expanding third-generation Medtronic CoreValve prosthesis (Medtronic) at the Heart Center Bonn and were included in this prospective registry. The decision for TAVI was made by the local Heart Team. The study was approved by the local ethics committee of the University of Bonn (no. 187/10), and all patients provided written informed consent.
All TAVI procedures were performed with biplane fluoroscopy under conscious sedation (except for the patients with transsubclavian [n=2] and transaortic [n=5] access). Therefore, the valve implantation itself was predominantly guided by angiographic control, while transoesophageal echocardiography (TEE) was only used to elucidate the aetiology of paravalvular AR in patients with more than mild AR and/or an AR index <25. From June 2011 onwards, all TAVI patients at the Heart Center Bonn were treated according to a multimodal algorithm, which we recently proposed31,32. After valve deployment, the degree of PVL was routinely assessed by aortic root angiography (30 mL contrast dye at a flow rate of 14 mL/s) according to the visually estimated density of opacification of the left ventricle into three degrees adapted from the VARC-2 criteria: mild (reflow of contrast in the outflow tract and middle portion of the LV but clearing with each beat), moderate (reflow of contrast in the whole left ventricular cavity with incomplete washout in a single beat and faint opacification of the entire LV over several cardiac cycles), and severe (opacification of the entire LV with the same intensity as in the aorta and persistence of the contrast after a single beat). In all patients, a 5 Fr pigtail catheter was placed 2 to 3 cm above the valvular plane.
In all patients, haemodynamics were assessed and the calculation of the AR index was performed to quantify the extent of PVL more precisely and to have a point of reference before corrective measures were taken. The dimensionless AR index is calculated according to the following formula: ([RRdia–LVEDP]/RRsys)×100 approximately five to 10 minutes after valve deployment or corrective measure to prevent confounding by an increased LVEDP due to myocardial ischaemia and/or diastolic dysfunction after rapid pacing and BPD. The AR index should be determined as the mean value over several cardiac cycles (especially in patients suffering from atrial fibrillation) with a heart rate of 60-80 bpm and without extrasystolic beats, since, with increasing heart rate and shortened duration of the diastole, the diastolic pressure in the aorta also increases and might thereby lead to a false negative AR index above the cut-off of 25. If the heart rate is below 60 due to high-degree atrioventricular block, pacing with a heart rate of 60-70 bpm is recommended for the assessment of the AR index31.
In patients with more than mild angiographically detected PVL and/or an AR index <25, PVL was evaluated by echocardiography, preferably TEE, to elucidate the cause of PVL and its mechanism if not obvious on fluoroscopy (implantation depth, prosthesis frame expansion). In addition, to assess prosthesis frame expansion on fluoroscopy, an extreme RAO cranial C-arm angulation was used to assure orthogonal projection of the CoreValve. This multimodal algorithm was used to identify patients who needed corrective measures such as BPD or ViV implantation to reduce the degree of paravalvular AR13,31. In patients with a proper implantation depth of the THV but suboptimal frame expansion, BPD was performed to obtain a better expansion of the prosthesis stent frame and a better sealing of the paravalvular space. In case of too shallow or too deep positioning of the THV or when BPD did not improve PVL, ViV implantation was considered31,32.
The primary endpoint of our study was the change of the AR index by BPD or ViV implantation for the reduction of PVL in patients undergoing TAVI with the use of the self-expanding CoreValve prosthesis. Secondary endpoints were the severity of PVL defined according to the VARC-2 criteria, all-cause mortality at one year, and stroke rate during the index hospitalisation.
Statistical analysis
Data are presented as mean±standard deviation if normally distributed or as median and interquartile range if not normally distributed. Continuous variables were tested for normal distribution with the use of the Kolmogorov-Smirnov test. Categorical variables are given as frequencies and percentages. For continuous variables, a Student’s t-test was performed for comparison between two groups. When comparing more than two groups, ANOVA or the Kruskal-Wallis test was used. For categorical variables, the χ2 test was used for further analysis. Survival according to the need for BPD or ViV implantation was determined by using the Kaplan-Meier method. Statistical significance was assumed when the null hypothesis could be rejected at p<0.05. Statistical analyses were conducted with PASW Statistics, version 18.0.3 (IBM Corporation, Somers, NY, USA) and MedCalc, version 11.6.1.0 (MedCalc Software, Mariakerke, Belgium).
The investigators initiated the study, had full access to the data, and wrote the manuscript. All authors vouch for the data and analysis.
Results
BASELINE CHARACTERISTICS
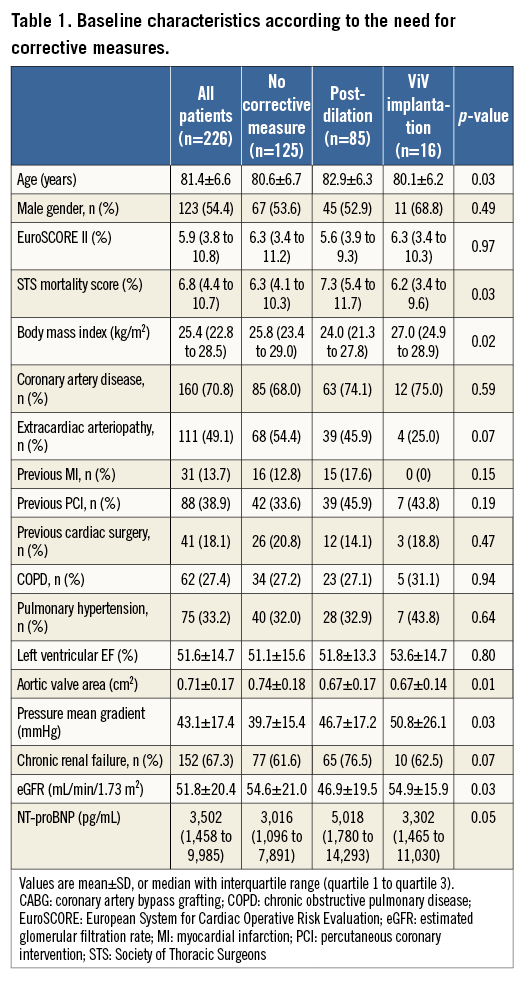
A total of 226 consecutive patients with a median EuroSCORE II of 5.9 (3.8 to 10.8), reflecting an increased risk for SAVR, underwent TAVI in this prospective study. Baseline characteristics are summarised in Table 1. Patients were subdivided into three groups: patients without the need for corrective measures after initial deployment of the self-expanding THV (n=125) and those who had to undergo either BPD (n=85) or ViV implantation (n=16) due to significant PVL.
Patients with the need for BPD were significantly older, had higher STS scores, smaller aortic valve areas (AVA), higher mean pressure gradients, and higher NT-proBNP levels than patients without the need for corrective measures. ViV implantation patients had smaller AVAs and higher mean pressure gradients than patients without any intervention (Table 1).
PERIPROCEDURAL CHARACTERISTICS
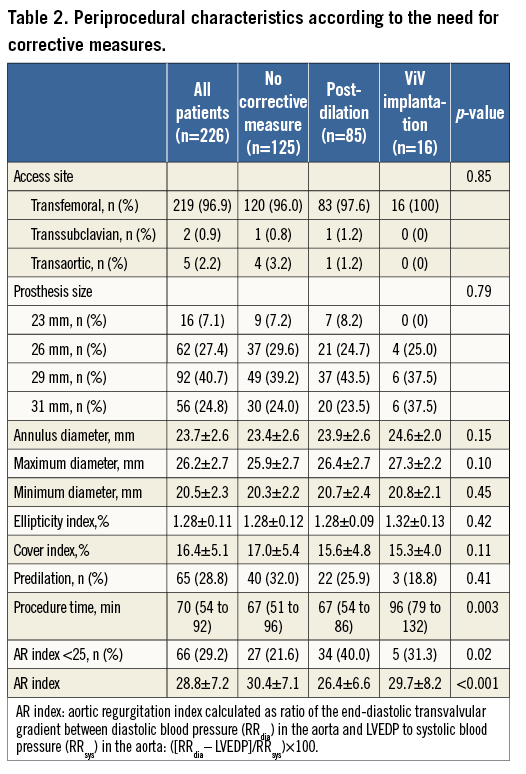
Periprocedural characteristics of the study cohort are summarised in Table 2. The transfemoral route was used in 96.9% of the study cohort. An association of BPD or ViV implantation with prosthesis size, annulus dimensions, and cover index was not found. More importantly, the rate of predilation was not different between patients with BPD or ViV implantation and those without corrective measures. The procedure time was significantly longer in patients undergoing valve-in-valve implantation.
CORRECTIVE MEASURES FOR THE REDUCTION OF PARAVALVULAR LEAKAGE
Following deployment of the CoreValve prosthesis, the severity of PVL was evaluated angiographically according to the visually estimated density of opacification of the left ventricle (Figure 1). No or mild AR was found in 46 patients (20.4%) and 83 patients (36.7%), respectively. However, a total of 97 patients (42.9%) suffered from more than mild PVL: moderate AR was detected in 69 patients (30.5%) and severe AR in 28 (12.4%).
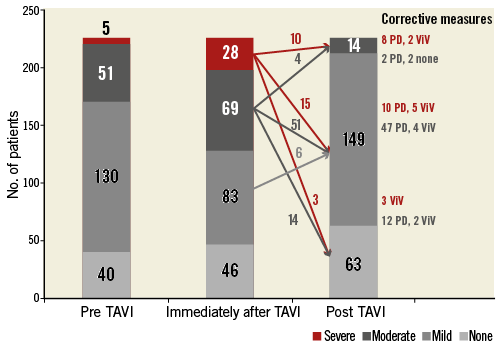
Figure 1. The severity of paravalvular leakage before and immediately after valve deployment and at the end of the TAVI procedure after corrective measures.
Due to significant PVL after initial deployment of the THV as detected by angiography in combination with the AR index, 44.7% (101/226 patients) underwent corrective measures for the reduction of paravalvular leakage (six patients without predilation who suffered from mild-to-moderate PVL underwent BPD due to significant frame underexpansion combined with an AR index <25). BPD was performed in 85 patients and ViV implantation in 16 patients, respectively. PVL reduction ≥1 degree was achieved in all patients with more than mild PVL so that no patient suffered from severe paravalvular AR. Finally, only 14 patients (6.2%) suffered from moderate paravalvular AR after these correction manoeuvres.
AR INDEX BEFORE AND AFTER CORRECTIVE MEASURES
Immediately following valve deployment, we determined an AR index of 20.2±11.9 in patients with moderate and of 15.5±6.5 in those with severe PVL. By the application of corrective measures for PVL reduction, the AR index could be increased to 26.3±5.1 (p=0.003) and 26.3±8.1 (p<0.001) in these patients, respectively. Figure 2 shows that both corrective measures were effective: BPD increased the AR index from 19.1±11.0 to 25.9±5.8 (p<0.001) and ViV implantation from 17.6±6.4 to 29.5±9.1 (p=0.008).
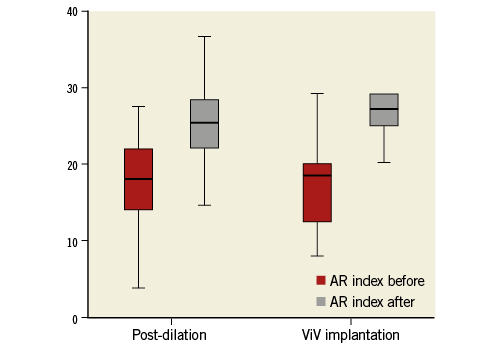
Figure 2. Aortic regurgitation index in patients with more than mild paravalvular leakage before and after balloon post-dilation (BPD) and valve-in-valve (ViV) implantation.
CLINICAL OUTCOMES AFTER POST-DILATION OR VALVE-IN-VALVE IMPLANTATION
Clinical outcomes were not different between patients with or without corrective measures (Table 3): we found similar one-year mortality rates (21.6% vs. 17.6% vs. 25.0%; p=0.69) (Figure 3). Importantly, we did not find an increased stroke rate in patients after BPD or ViV implantation (2.4% vs. 2.4% vs. 0%; p=0.82). We found a higher rate of remaining more than mild PVL and AR indices <25 in patients after corrective measures, since only in these patients with significant PVL did corrective measures have to be taken. The 66 patients with a residual AR index <25 had a significantly higher one-year mortality compared to patients with an AR index >25 (31.8% vs. 15.6%; p=0.001). Residual more than mild PVL in 14 patients (50.0% vs. 17.6%, p=0.001) was also associated with a worse outcome than no or only mild PVL during the first year of follow-up after TAVI.
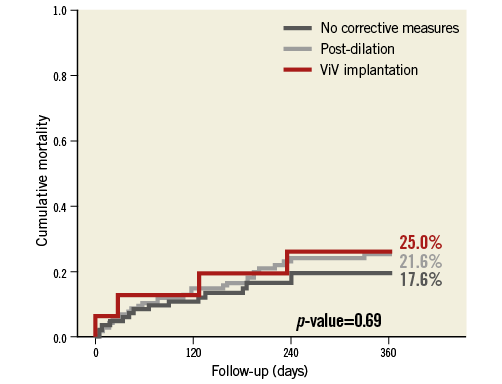
Figure 3. One-year mortality according to the need for balloon post-dilation or valve-in-valve implantation compared to patients without the need for corrective measures.
Discussion
Our study demonstrates that both BPD and ViV implantation can effectively and safely reduce the severity of PVL in self-expanding THVs when the initial result after deployment of the THV is not acceptable (Figure 4). The dimensionless AR index is a useful parameter as a benchmark before any intervention to quantify the success of corrective measures thereafter (in addition to imaging modalities). Moreover, reasonable use of BPD as a corrective measure for more than mild PVL and ViV implantation as a bail-out option for a suboptimally placed valve were both not associated with an increased risk for cardiovascular complications such as stroke, coronary obstruction, or an increased one-year mortality risk.
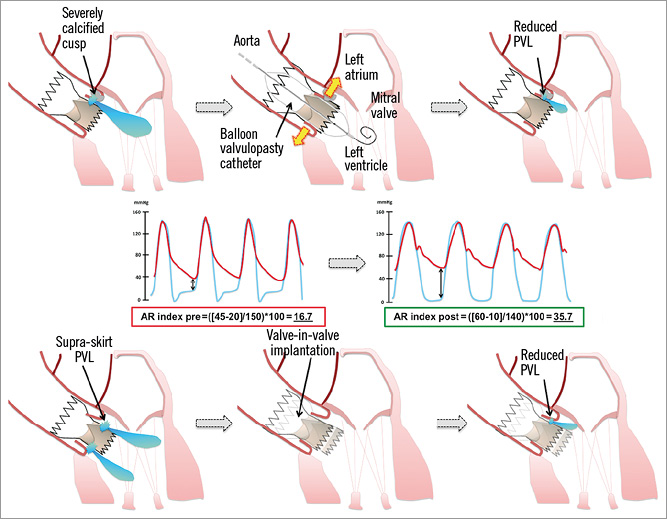
Figure 4. The effect of balloon post-dilation or valve-in-valve implantation on the reduction of paravalvular leakage (PVL) and haemodynamics as assessed by the aortic regurgitation index.
BALLOON POST-DILATION
BPD has been intuitively utilised to reduce the degree of PVL after valve deployment during the TAVI procedure, especially in cases with frame underexpansion due to aortic root calcifications. So far, two studies with a post-dilation rate of 28 to 41% evaluated the effectiveness and safety of BPD in balloon-expandable THVs3,28. In two smaller series with use of the self-expanding CoreValve, up to 30% of the patients had to be post-dilated with a balloon aortic valvuloplasty catheter26,27. BPD has been predominantly performed due to suboptimal frame expansion attributable to incomplete circumferential apposition of the prosthesis to the native aortic annulus3-10. In our study cohort, BPD was applied in 38% of the patients undergoing TAVI with the use of the CoreValve prosthesis. Importantly, underexpansion of the prosthesis stent frame occurred independently from predilation of the native aortic valve in our series. Since patients with BPD had smaller AVAs, higher mean pressure gradients, and higher NT-proBNP levels, one might hypothesise that these patients suffered from a more advanced stage of aortic stenosis.
Several studies have hypothesised that the use of BPD might be associated with a higher rate of cardiovascular complications, such as prosthesis migration, conduction disturbances, annulus rupture, coronary obstruction, and cerebral embolism leading to stroke1,3,28,33,34. In contrast, our study demonstrated that BPD is not only an effective but also a safe option for PVL reduction in self-expanding THVs, when the following recommendations are taken into account. The size of the balloon for post-dilation should be adjusted to the aortic annulus dimension and should not exceed the mean diameter of the native aortic valve. For the CoreValve prosthesis, a straight valvuloplasty balloon with a maximum diameter up to 22, 25, 28, and 30 mm is recommended for the 23, 26, 29, and 31 mm CoreValve, respectively31.
VALVE-IN-VALVE IMPLANTATION
Implantation of a second prosthesis within the first has been suggested as a bail-out manoeuvre to overcome the suboptimal too high or too deep initial device positioning ultimately to minimise the degree of significant paravalvular leakage31.
In earlier studies with the self-expanding CoreValve prosthesis, ViV implantation became necessary in up to 3% of the patients. The first prosthesis was then pulled with a snare catheter into the ascending aorta before deploying the second in the correct anatomic position35,36. In studies using the balloon-expandable Edwards prosthesis, ViV was also performed in approximately 3% of the patients by direct implantation of a second THV without prior retraction of the initially implanted valve37-39.
In our study cohort, using the CoreValve prosthesis, ViV implantation was applied in 7% of the TAVI patients but –in contrast to the above-mentioned studies– the originally misplaced CoreValve prosthesis was left in situ and was not repositioned using a snare catheter to retract the THV from the aortic annulus into the ascending aorta prior to the deployment of the second CoreValve prosthesis. We abandoned this strategy because snaring of an already placed valve is more or less unpredictable. Although this resulted in a significant overlap of the pericardial skirts of both prostheses (for PVL reduction), we did not find a higher incidence of periprocedural myocardial infarction due to coronary artery obstruction or cardiac conduction abnormalities with consecutive permanent pacemaker implantation, as hypothesised by prior studies35,38. Thus, our study also demonstrates that ViV implantation leaving the first CoreValve in place without snaring is a safe option. Not surprisingly, only the procedure time was significantly prolonged compared to a TAVI procedure without any corrective measure or BPD only.
AORTIC REGURGITATION INDEX AS BENCHMARK
The aortic regurgitation index is an objective and reproducible parameter for the assessment of the severity of PVL during the TAVI procedure and should be used in addition to imaging modalities such as angiography or TEE13,31. After validation in an independent cohort, an AR index cut-off value of 25 has been shown to be an independent predictor of the one-year mortality risk after TAVI. Although an AR index <25 might partly be caused by the procedure-related elevated LVEDP, diastolic dysfunction, or a low diastolic aortic blood pressure before TAVI, this AR index cut-off value has a negative predictive value of 95% to 100% for the occurrence of more than mild PVL. In addition, the AR index depends on the heart rate. Therefore, it is recommended to perform the measurements approximately five to 10 min after valve deployment to prevent confounding by an increased LVEDP due to myocardial ischaemia and/or diastolic dysfunction after rapid pacing and balloon valvuloplasty as a mean value over several cardiac cycles (especially in patients with atrial fibrillation) with a heart rate of 60 to 80 beats/min and without extrasystolic beats. Increased heart rate and shortened duration of the diastole result in a false negative AR index31. However, this applies to all imaging modalities in use.
Our study demonstrates for the first time that the AR index may also serve as a point of reference before corrective measures are applied, and, decisively, the AR index helps to predict and to quantify the success of these interventions. Both BPD and ViV implantation increased the AR index significantly.
Study limitations
Sample size and the single-centre character are limitations of our study. For further verification and generalisation of our results, larger studies are needed, especially to confirm the safety and efficacy of BPD in routine use. Furthermore, our findings are based on the experience with the self-expanding CoreValve prosthesis, which may limit their applicability to other transcatheter heart valves. Finally, the impact of balloon dilation for valve deployment or that of post-dilation on the long-term durability of THV should be elucidated in ongoing large registries.
As already mentioned above, the AR index might be confounded by high systemic blood pressure, concomitant diastolic dysfunction, myocardial ischaemia during and after valve deployment or BPD as well as the use of catecholamines during the procedure, something which might lead to an increase of LVEDP leading to false positive AR indices. Likewise, the heart rate and its undeniable influence on the diastolic aortic blood pressure affects the AR index. To prevent confounding and ensure reproducible haemodynamic measurements, one has to follow the recently published and above-mentioned recommendations for the assessment of the AR index31.
Conclusions
Balloon post-dilation and valve-in-valve implantation are safe and effective treatment options to reduce the severity of paravalvular leakage following deployment of the self-expanding CoreValve prosthesis. To assess the success of BPD or ViV implantation objectively, the aortic regurgitation index can be used as a benchmark before and after the intervention.
| Impact on daily practice Paravalvular leakage (PVL) as a procedure-related shortcoming of TAVI is caused by incomplete circumferential apposition of the prosthesis stent frame at the level of the aortic annulus. Balloon post-dilation and valve-in-valve implantation are safe and effective treatment options to reduce the severity of paravalvular leakage following deployment of the self-expanding CoreValve prosthesis. To assess the success of BPD or ViV implantation objectively, the aortic regurgitation index can be used as a benchmark before and after the intervention. |
Conflict of interest statement
J.M. Sinning, E. Grube, G. Nickenig, and N. Werner receive research grants and speaker honoraria from Medtronic and Edwards Lifesciences. E. Grube works as a proctor for Medtronic. The other authors have no conflicts of interest to declare.

