
Abstract
Aims: The aims of this study were to determine the appropriateness of permanent pacemaker implantation (PPI) after TAVI through an analysis of PM dependency at follow-up, and to assess long-term outcomes of patients undergoing PPI after TAVI.
Methods and results: From June 2007 to February 2018, 1,116 consecutive patients without prior PM underwent TAVI in our institution. We assessed the incidence and predictors of PM dependency of patients who underwent PPI within 30 days, and also the six-year outcomes among patients who did not undergo PPI at 30 days. At 30 days, PPI was reported in 145 patients (13.0%). Rates of PM dependency were 35.7%, 35.8% and 33.3% at 1, 6 and 12 months, respectively. Analysing PPI timing, implantation on day 1 was found to be a predictor of PM dependency at six months (OR 20.7 [95% CI: 3.4-126.7]; p=0.001) and 12 months (OR 7.5 [95% CI: 1.4-40.2]; p=0.019). An interaction between PM dependency and the presence of baseline right bundle branch block (RBBB) at six months (pinteraction=0.024) and 12 months (pinteraction=0.028) was reported when PPI was performed on the same day as TAVI. At six years, patients who received a PM at 30 days showed a higher all-cause death rate (KM estimate 41.7% vs 57%; plog-rank=0.034).
Conclusions: Among patients receiving PPI after TAVI, PM dependency rates were about 33-36% at one year. Patients with a baseline RBBB undergoing PPI at day 0 or at day 1 when severe CDs persisted for 24 hours after TAVI, irrespective of baseline CDs, had a higher chance of being PM-dependent at follow-ups. Finally, PPI after TAVI was associated with increased six-year mortality.
Introduction
Transcatheter aortic valve implantation (TAVI) is currently a valid and less invasive alternative to conventional surgical aortic valve replacement (SAVR) for patients with severe, symptomatic aortic stenosis (AS)1. On the basis of the favourable outcomes of recent randomised clinical trials conducted in high- and intermediate-risk populations1, TAVI is progressively being offered to younger and lower-risk patients. High-degree atrioventricular conduction disturbances (AVCD) are among the most important issues relating to TAVI that need to be investigated further before expanding the indications2,3. Left bundle branch block (LBBB) and complete atrioventricular block (AVB) are the most frequent conduction disturbances (CDs)4,5. Although a number of predictors of permanent pacemaker implantation (PPI) after TAVI have been established in many studies6, it remains poorly investigated whether permanent pacemakers (PM) implanted in this setting are really necessary. The aims of this prospective study were to determine the appropriateness of PPI after TAVI through an analysis of PM dependency at follow-up, and to assess long-term outcomes of patients undergoing PPI after TAVI.
Methods
STUDY DESIGN AND POPULATION
This is a single-centre, prospective study including consecutive patients who underwent TAVI in our institution, receiving most of the devices available on the market (Edwards SAPIEN XT and SAPIEN 3 [Edwards Lifesciences, Irvine, CA, USA], CoreValve®, Evolut™ R and Evolut™ PRO [Medtronic, Minneapolis, MN, USA], ACURATE neo™ TF [Boston Scientific, Marlborough, MA, USA], and Portico™ [St. Jude Medical, St. Paul, MN, USA]).
Patients with a previous PM implanted were excluded from the present analysis (N=129, 10.2%). The study flow chart is shown in Figure 1.
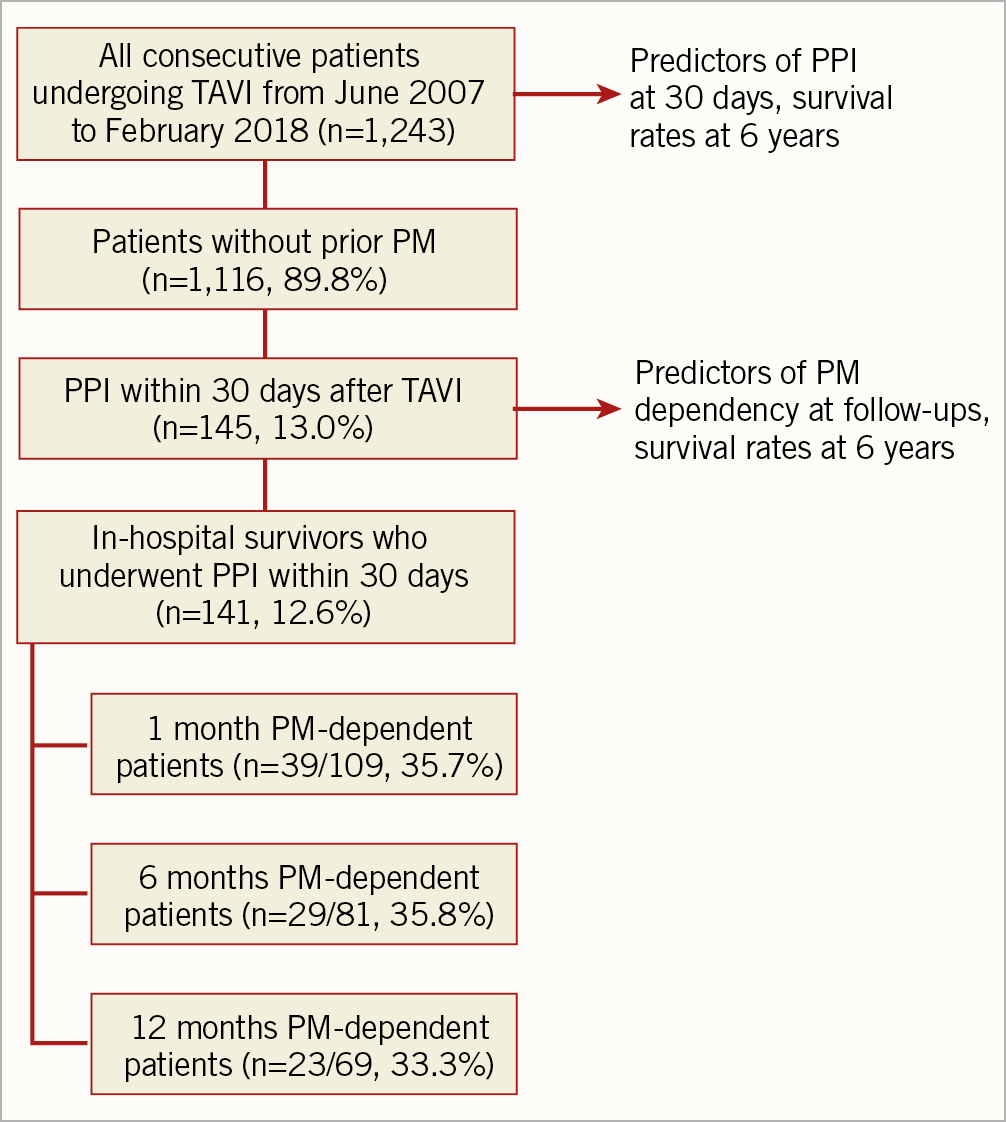
Figure 1. Study flow chart.
CRITERIA FOR PPI AFTER TAVI
All PPI in our cohort followed the indications of the available European guidelines7. Patients with persistent high-degree conduction disturbances underwent PPI within 24 hours. The reasons for postponing PPI after 24 hours despite the occurrence of high-degree AVCD were transient or intermittent blocks.
Device programming was standardised in all patients. Single-chamber PMs were programmed to a VVI(R) mode and dual-chamber PMs to the DDD(R) mode, activating proprietary algorithms that minimise ventricular pacing, whenever applicable. The lower rate limit was set to 60 beats per minute (bpm) in all devices.
FOLLOW-UP DATA
For the purpose of the present analysis, we performed clinical visits up to six years and pacemaker device controls at 1, 6 and 12 months after the procedure. Furthermore, we performed echocardiographic evaluation of left ventricular ejection fraction (LVEF) at 30 days, 1, 3 and 5 years after TAVI, as per standard practice.
STATISTICAL ANALYSIS
Continuous variables were reported as mean±standard deviation (SD). Categorical variables were reported as number and percentage. Two-step analysis was used to assess predictive factors. First, a single logistic regression was performed. Variables with a p-value <0.10 were entered into a multiple logistic regression analysis. Results were reported as odds ratio (OR) with a 95% confidence interval (CI). Survival curves for the outcomes of interest were plotted according to the Kaplan-Meier (KM) method. The log-rank sum test was used to compare estimated rates. All statistical tests were performed two-tailed, and a significance level of p<0.05 was considered to indicate statistical significance.
A sensitivity analysis was performed excluding patients receiving the Lotus™ device (Boston Scientific).
The statistical software package SPSS, Version 24.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
All outcomes were reported according to the Valve Academic Research Consortium-2 definitions8. Transcatheter aortic valve (TAV) oversizing was calculated as area oversizing (%) = (TAV area/annulus area – 1)×1009. Pacemaker dependency was defined as the absence of an escape or intrinsic rhythm for 30 seconds during temporary back-up pacing at a rate of 30 bpm10.
Results
POPULATION
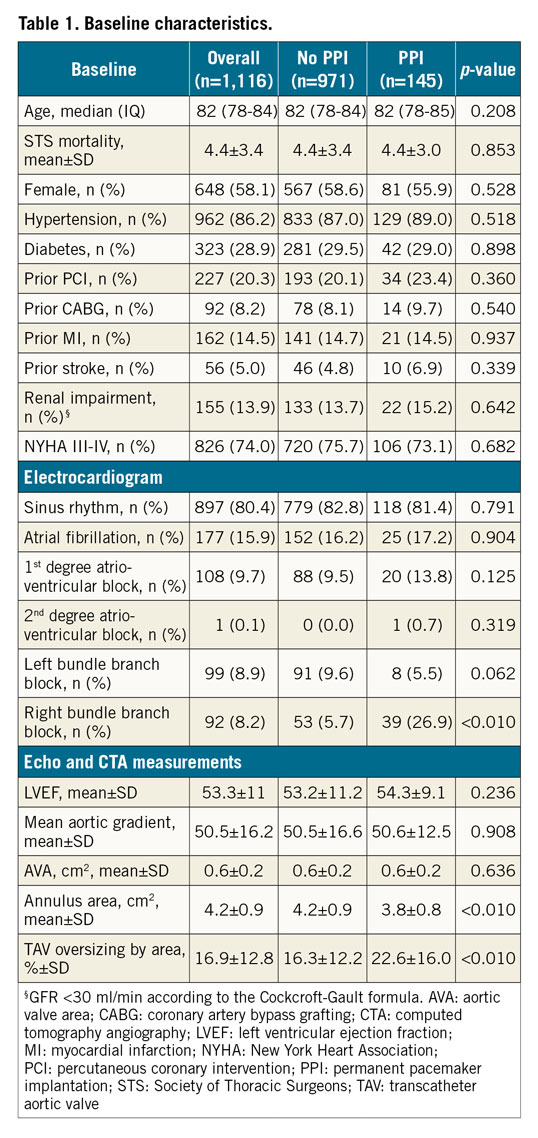
A total of 1,116 patients with a mean age of 80.9±5.3 years were considered for the present analysis. At 30 days following TAVI, 145 patients (13.0%) had a permanent PM implanted. Baseline demographic, clinical, electrocardiographic, echocardiographic and computed tomography characteristics of the study population are summarised in Table 1. All patients had severe symptomatic AS, with a mean transaortic gradient of 50.5±16.1 mmHg and a mean aortic valve area of 0.6±0.2 cm2. Most patients were in sinus rhythm (80.4%), 8.9% of them had a left bundle branch block (LBBB) and 8.2% of them had a right bundle branch block (RBBB) at baseline. The predicted 30-day mortality, as assessed by the Society of Thoracic Surgeons (STS) mortality score, was 4.4±3.4%. Almost 3/4 of patients (73.8%) were in New York Heart Association functional Class III or IV before the procedure. Patients who received a permanent PM within 30 days more commonly had an RBBB at baseline (26.9% vs 5.7%, p<0.01) and received a bioprosthesis with a higher degree of oversizing (22.6% vs 16.3% by area, p<0.01).
PROCEDURAL AND 30-DAY OUTCOMES
Procedural data are shown in Supplementary Table 1. Most of the procedures were performed through the transfemoral access (97%) and under local anaesthesia (97.3%). Either self-expanding (CoreValve [36.3%], Evolut R [24.3%], Evolut PRO [1.4%], Portico [1.6%], ACURATE neo TF [8.9%]), or balloon-expandable (SAPIEN XT [11.3%], SAPIEN 3 [15.9%]) TAVs were used. Balloon predilatation was performed in 947 patients (84.9%), more commonly in patients who underwent PPI within 30 days (91.7% vs 83.8%, p=0.003). Device success was obtained in 987 patients (89.2%). Patients undergoing PPI had a lower rate of optimal device implantation depth according to manufacturers’ indications (87.6% vs 94.9%, p<0.05).
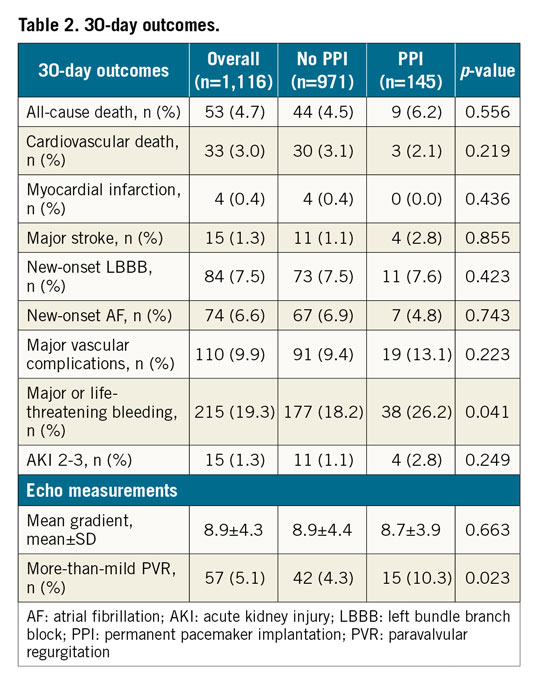
The 30-day outcomes are listed in Table 2. Overall, the 30-day mortality rate was 3.9%. Major stroke and major vascular complications were reported in 1.3% and 9.9% of patients, respectively. Patients who received a PM at 30 days had a higher rate of more-than-mild paravalvular regurgitation (PVR) (10.3% vs 4.3%, p<0.05).
PPI AND PM DEPENDENCY
PPI and PM dependency outcomes are reported in Table 3. At a median of 23.9 months (IQR 9.4-44.6), a total of 180 patients had received a PM. One hundred and thirty-eight (12.4%) patients underwent PPI during the index hospitalisation, while seven patients (0.6%) were re-hospitalised within 30 days due to high-degree AVB.
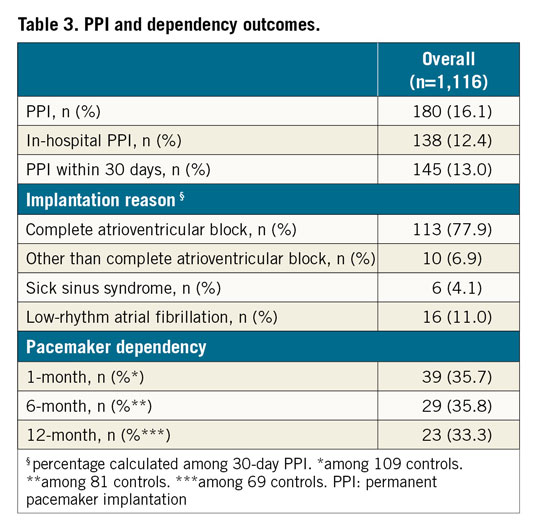
Among the 145 patients receiving a PM within 30 days, 113 (77.9%) had complete AVB, 10 (6.9%) had symptomatic advanced incomplete AVB, 6 (4.1%) were affected by sick sinus syndrome (SSS) and 16 (11.0%) had atrial fibrillation with low ventricular response. In this group, the rate of PM dependency was 35.7%, 35.8% and 33.3% at 1, 6 and 12 months, respectively.
PPI on day 1 was found to be a predictor of PM dependency both at 6 months (OR 20.7 [95% CI: 3.4-126.7]; p=0.001) and 12 months (OR 7.5 [95% CI: 1.4-40.2]; p=0.019), with a trend towards statistical significance at 1 month (OR 3.1 [95% CI: 1.0-9.6]; p=0.056] (Figure 2). PPI the same day as TAVI (persistent high-degree CDs) or at day 2 and days 3-30 (transient or intermittent CDs) were not found to be associated with PM dependency at follow-up.
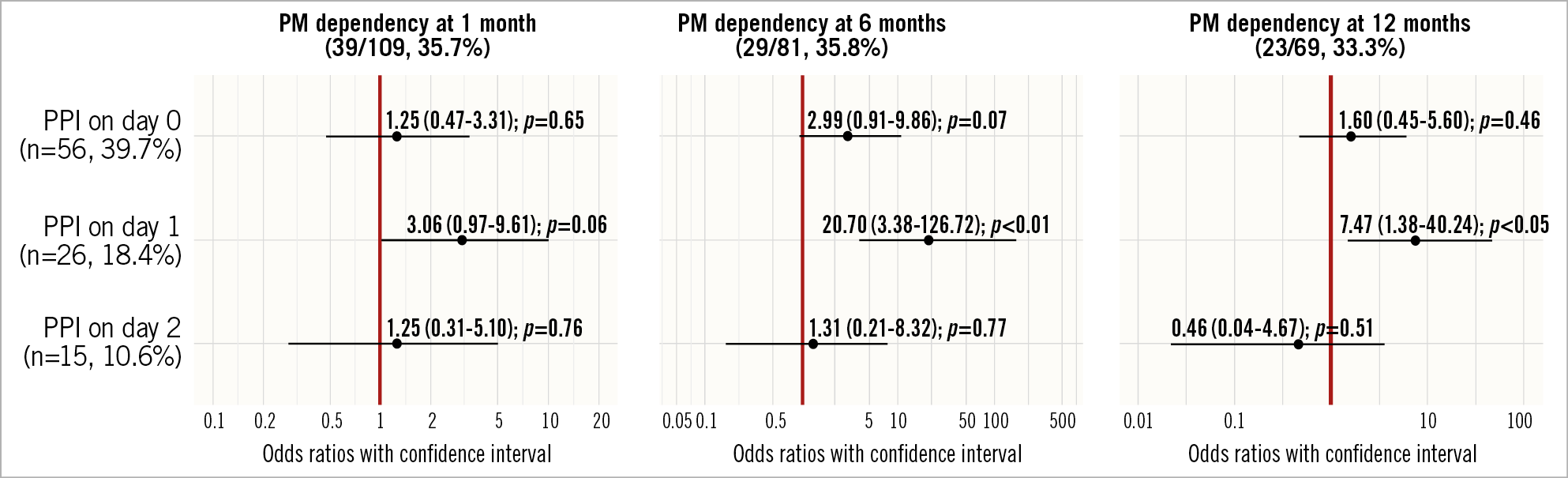
Figure 2. PPI timing predictors of PM dependency on follow-ups.
In an exploratory analysis, we assessed the PM dependency at 30 days, 6 months and 12 months in a pre-specified subgroup of patients with baseline RBBB (Supplementary Table 2-Supplementary Table 4). An interaction between PM dependency and the presence of baseline RBBB at six months (pinteraction=0.024) and 12 months (pinteraction=0.028) was reported when PPI was performed on the same day as TAVI. No correlations at day 1, day 2 and days 3-30 were found.
Evaluating predictors of PPI within 30 days, at multivariate analysis a higher TAV oversizing (16.3% vs 22.6% by area; OR 1.040 [95% CI: 1.015-1.065]; p<0.01) and pre-existing RBBB (26.9% vs 5.7%; OR 4.505 [95% CI: 1.976-10.269]; p<0.01) were associated with increased risk of PPI (Figure 3).
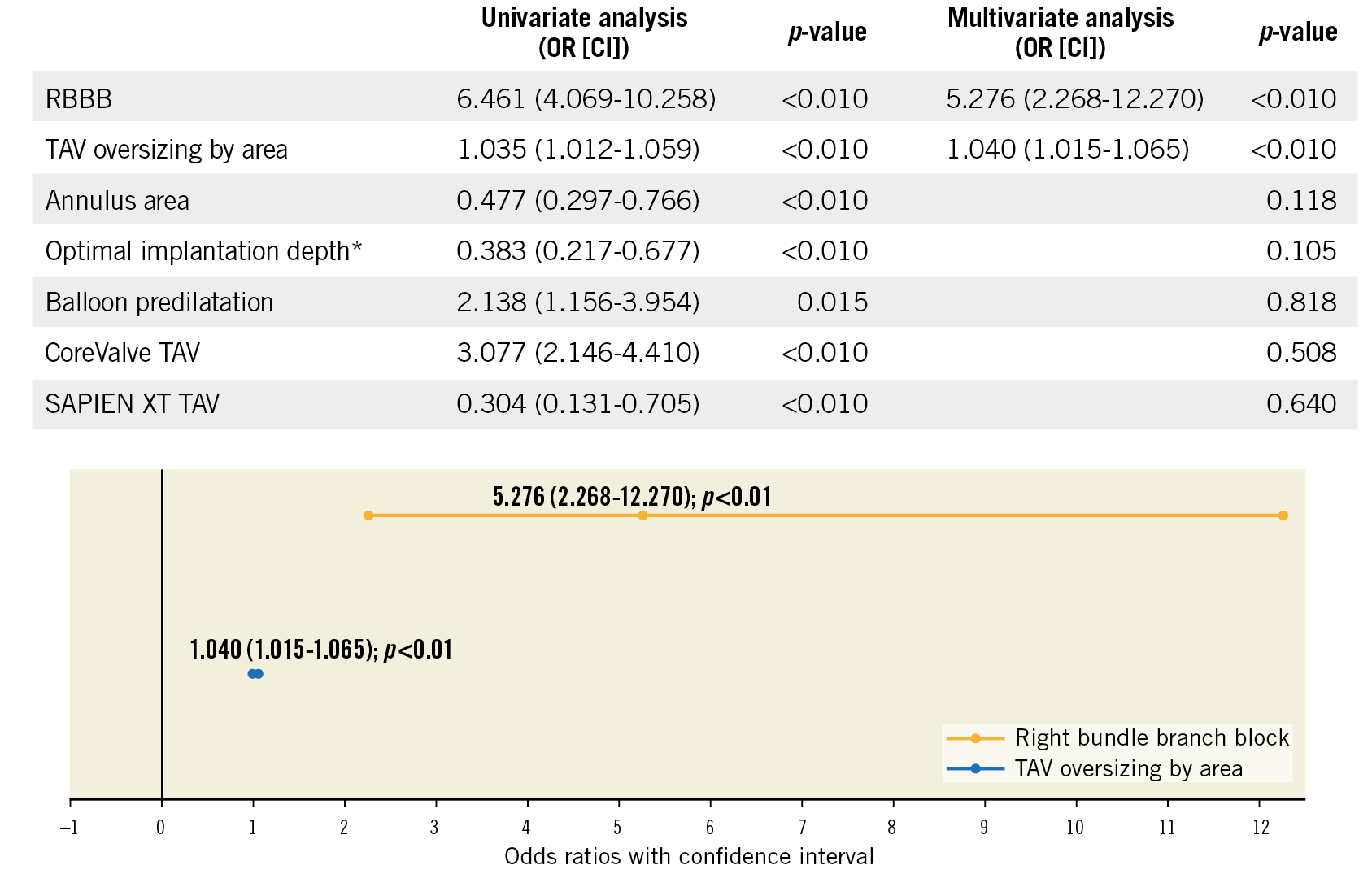
Figure 3. Multivariate logistic regression analysis with pacemaker implantation <30 days. According to manufacturers’ instructions for use (IFU).
LONG-TERM OUTCOMES
Long-term outcomes up to six years are reported in Figure 4. At six-year follow-up, patients who received a PM at 30 days showed a higher overall mortality rate (KM estimate 41.7% vs 57%; plog-rank=0.034) (Figure 4A). In an exploratory analysis, no interaction effect of more-than-mild PVR at one month over the association between PPI within 30 days and overall mortality was reported at six years (pinteraction=0.824).
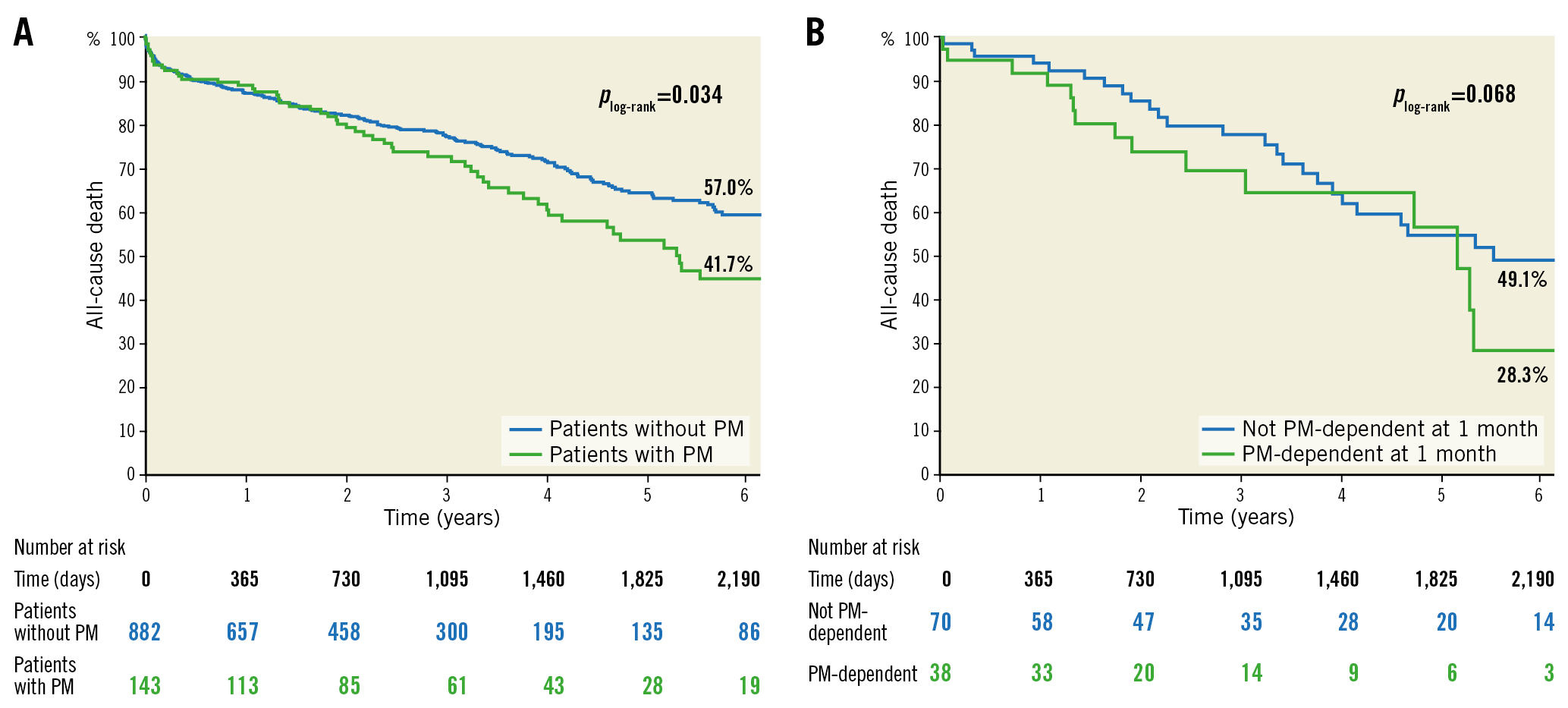
Figure 4. Kaplan-Meier survival estimates at six years. A) KM curves of patients who underwent PPI within 30 days from TAVI and those who did not. B) KM curves of patients who were PM-dependent at one-month follow-up and those who were not.
Analysing in-hospital survivors who underwent PPI within 30 days from TAVI, PM-dependent patients at one month showed a higher overall mortality rate compared to non-dependent patients (KM estimate 28.3% vs 49.1%; plog-rank=0.068) at six years (Figure 4B).
Finally, on transthoracic echocardiography, patients undergoing PPI within 30 days from TAVI showed a reduced LVEF at 30-day (53.6% vs 56.2%, p=0.01), 1-year (54.2% vs 57.7%, p<0.01), 3-year (51.1% vs 56.2%, p<0.05) and 5-year (48.0% vs 55.4%, p=0.10) follow-up (Supplementary Table 5).
Discussion
PPI after TAVI and its consequences remain an issue of debate in a real-world setting. TAVI has been considered for lower-risk and younger patients during recent years, but the impact of prostheses on the conduction system and the consequent risk of PPI necessity is a factor to take into account in the Heart Team decision-making process. Although the clinical consequences of a PPM after TAVI remain the subject of ongoing debate, the deleterious effects of CDs and a decrease in left ventricular function induced by a right ventricle-based paced rhythm are supported by solid evidence in other clinical settings11,12,13.
The main findings of our analysis were the following. 1) Among patients undergoing PPI within 30 days after TAVI, PM dependency at follow-up was found in approximately 1/3 of patients, with consistent rates both at six and 12 months. 2) Patients had higher chances of being PM-dependent if PPI occurred at day 1 after TAVI (severe conduction disturbance persisting for 24 hours) or even at day 0 if baseline RBBB was present. Patients with severe CDs and no RBBB undergoing early (same day) PPI, and patients with intermittent CDs undergoing PPI after day 1 had a higher possibility of being found to be PM non-dependent at follow-up. 3) PPI increased the overall long-term mortality rate after TAVI. Moreover, PM dependency at one month was demonstrated to have a negative impact on overall survival rates after TAVI.
PM DEPENDENCY AFTER TAVI
A few studies have reported on PM dependency after TAVI, exploring small samples of patients14,15,16. It is to be stressed that the definition of PM dependency itself is not uniform among studies; this makes a comparison complicated. In our study, we defined PM dependency as the absence of an escape or intrinsic rhythm for 30 seconds during temporary back-up pacing at a rate of 30 bpm.
At controls of the devices, patients undergoing PPI within 30 days after the procedure showed dependency rates of 35.7%, 35.8% and 33.3% at 1, 6 and 12 months, respectively. Nevertheless, PM dependency at controls was found not to be consistent for a certain percentage of patients, thus supporting the individual variability of infra-Hisian conduction system response on stimuli, as reported by Schernthaner et al17.
The only factor associated with PM dependency was found to be PPI on day 1 after TAVI, which in our practice was carried out in those patients with persistent high-degree CDs, whereas very early PPI (same day as TAVI) was not associated with PM dependency. This strongly supports the current ESC guidelines indication of a period of watchful waiting for any recovering of physiological impulse conduction after transient damage caused by the deployment of metal-framed TAVs. Nevertheless, we reported an interaction between RBBB at baseline and PM dependency at six and 12 months, with a higher rate of PM-dependent patients with RBBB at baseline when PPI was performed on the same day as TAVI. This supports the appropriateness of an immediate PPI after the procedure if a patient with a diseased right intraventricular conduction system develops a complete AVB, as a result of the injury of the remaining non-diseased conduction system due to the TAV implanted (Figure 5).
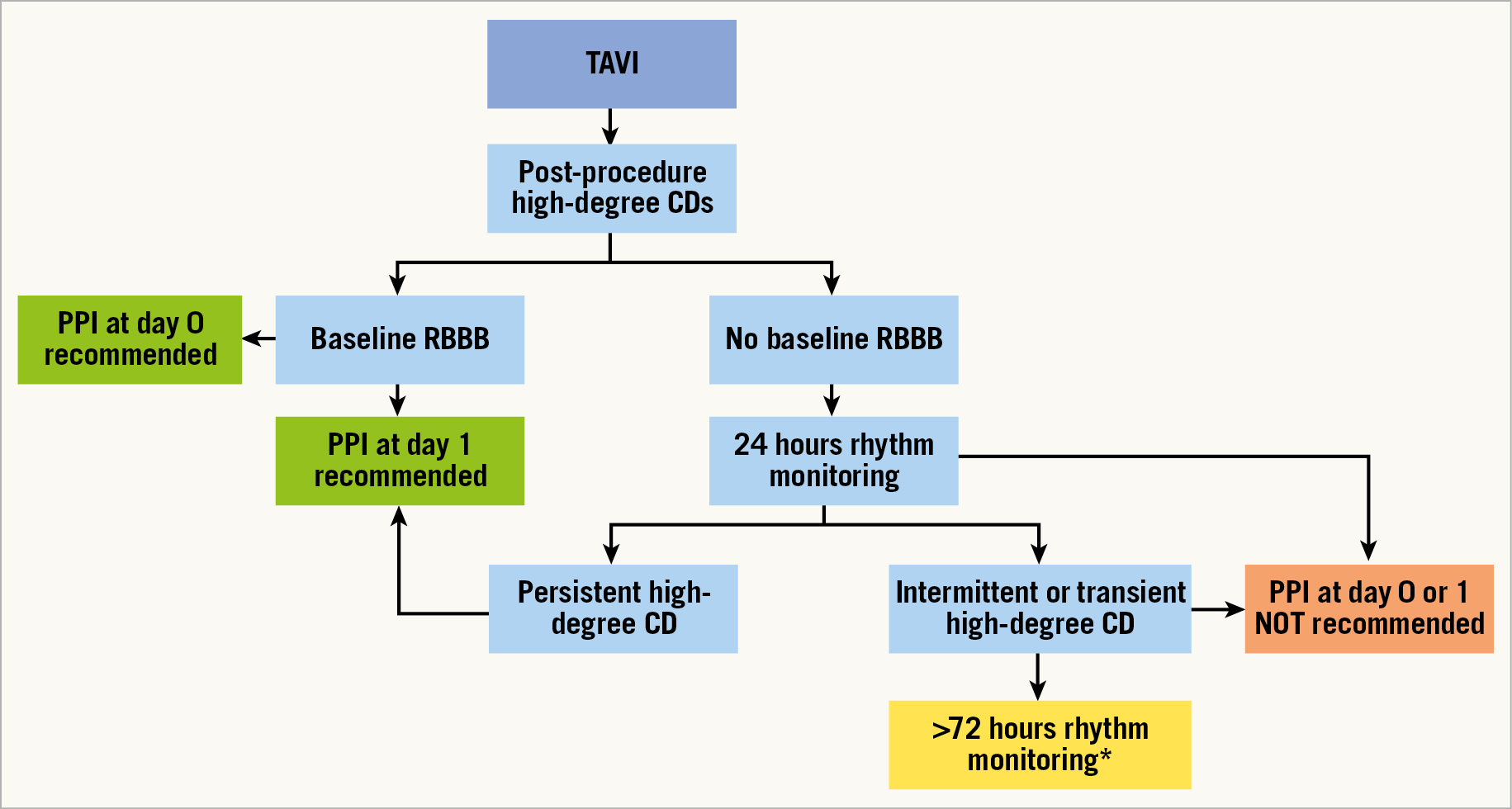
Figure 5. Timing recommendation for PPI after TAVI. *Timing of monitoring must be tailored according to each patient’s characteristics. CD: conduction disturbance; PPI: permanent pacemaker implantation; RBBB: right bundle branch block
Interestingly, patients who had the PM implanted at day 2 and days 3-30 after TAVI were mostly found not to be PM-dependent at follow-up. The explanation for this finding can be attributed to our timing of PPI in case of intermittent or transient high-degree AVB. In fact, these patients probably underwent too short a period of observation before PPI. According to these results, patients with transient episodes of high-degree AVB could benefit from a longer period of watchful waiting before PPI to avoid inappropriate implants, which would prolong postoperative in-hospital stay. However, the exact timing for waiting before PPI remains unknown, and the data collected in this study do not allow any recommendations to be made concerning this important issue.
PREDICTORS OF PPI AFTER TAVI
The close proximity between the aortic valve and the conduction system explains the genesis of periprocedural CDs during TAVI18. The interaction of the stent frame of a valve prosthesis may lead to direct trauma, compression, haemorrhage, ischaemia, or infarction of the conduction system, as demonstrated by necropsy studies19.
Furthermore, in a large meta-analysis involving 11,210 TAVI patients, Siontis et al demonstrated that baseline disorders of the conduction system are important predictors of PPI after the procedure20. In our study, RBBB was confirmed to be the strongest predictor of PM implantation after TAVI6. Once again, this highlights the impact on the left-side conduction system of TAVI devices and the consequent higher risk of developing a complete AVB with the necessity for permanent pacing if the right side of the conduction system is already involved.
Moreover, a higher degree of TAV oversizing was found to be related with PPI after the procedure21. This is reasonable due to the higher compression force of the device on the conduction system tissue, as a result of the radial force of a larger stent into a relatively smaller annulus.
IMPACT OF PPI AND DEPENDENCY ON SURVIVAL
As TAVI is being offered to younger and lower-risk patients, the impact of PM implantation and dependency on overall mortality is a matter of debate.
To date, the association of PM with midterm mortality remains controversial, which may reflect differences in PPI indications, PM dependency, and ventricular pacing rates across studies. Urena et al demonstrated that patients with a new PM experienced a decrease in LVEF at one year post TAVI22. It was observed that pacing dependency >40% is a predictor of the development of heart failure23,24. Ventricular dyssynchrony can cause reduced cardiac output, systolic and diastolic dysfunction, reduced myocardial perfusion, sympathetic activation, endothelial dysfunction, and long-term adverse structural changes – that is, “pacing-induced heart disease”11.
The latest report from the STS/TVT Registry encompassing 9,785 TAVI recipients demonstrated an increased risk in one-year overall mortality among patients who had a PM after TAVI (adjusted hazard ratio 1.31; 95% CI: 1.09-1.58; p=0.003)25. Nevertheless, an analysis by Buellesfeld et al26 revealed that the 12-month all-cause mortality rate was similar among patients without PM (18.0%), patients with PM before TAVI (22.9%), and patients with PM after TAVI (19.4%).
In our study, patients who underwent PPI within 30 days after the procedure showed a higher mortality rate and a reduced LVEF up to six-year follow-up. Moreover, PM dependency at one-month control was found to be associated with a higher estimated overall mortality rate at six years, although this did not reach statistical significance. We can speculate that the higher pacing rate of PM-dependent patients, as previously identified, impacts on myocardial function. This might lead to a higher mortality rate.
Limitations
Limitations of our analysis include the relatively small sample size and its single-centre nature. In addition, PQ and QRS durations at baseline and at follow-up were not investigated prospectively. PM dependency is a variable parameter at follow-up and the definition of dependency itself is not uniform among studies, thus rendering a comparison with other series complicated. However, we used the definition most frequently adopted in the literature. Furthermore, follow-up on pacemaker dependency was performed in a “survival cohort”, with death possibly exerting a competing risk that may have biased our results. Finally, the impact of PPI on late mortality was not adjusted for confounding variables and we cannot exclude that this relationship could be an epiphenomenon.
Conclusions
Among patients receiving PPI after TAVI, PM dependency rates were about 33-36% at one year. Patients experiencing severe CDs persisting for 24 hours had a higher chance of being PM-dependent if PPI was carried out at day 1 or on the same day as TAVI if baseline RBBB was present. Patients with severe CDs and no RBBB undergoing same-day PPI, and patients with transient or intermittent CDs undergoing PPI after day 1 had a higher possibility of being found to be PM non-dependent at follow-up. Finally, PPI after TAVI was associated with increased six-year mortality, particularly in patients who were PM-dependent.
|
Impact on daily practice On the basis of the favourable outcomes of recent randomised clinical trials conducted in high- and intermediate-risk populations, TAVI is progressively being offered to younger and lower-risk patients. High-degree atrioventricular conduction disturbances (AVCD) are among the most important issues of TAVI that need to be investigated further before expanding the indications. In our study, PM dependency rates were about 33-36% at one year among patients who underwent PPI within 30 days from TAVI. Patients experiencing severe CDs persisting for 24 hours had a higher chance of being PM-dependent if PPI was carried out at day 1 or on the same day as TAVI if baseline RBBB was present. Moreover, PM-dependent patients after TAVI seem to have a higher incidence of long-term mortality. Careful rhythm monitoring during the first hours after the procedure is of paramount importance for limiting PPI to those patients experiencing persisting high-degree CDs, whereas longer monitoring seems to be the optimal strategy for those with intermittent severe CDs. Nevertheless, further larger, longer-term analyses of the impact of PPI and PM dependency on overall survival after TAVI are necessary to support this strategy. |
Conflict of interest statement
M. Barbanti is a consultant for Edwards Lifesciences and is an advisory board member for Biotronik. C. Tamburino has received speaker honoraria from Medtronic, Abbott Vascular, Edwards Lifesciences and Boston Scientific. The other authors have no conflicts of interest to declare.

