
Abstract
Aims: This analysis aimed to evaluate the incidence and predictors of the need for permanent pacemaker (PPM) implantation following implantation of the repositionable and fully retrievable LOTUS Aortic Valve Replacement System.
Methods and results: The prospective, single-arm, multicentre REPRISE II study with extended cohort enrolled 250 symptomatic, high surgical risk patients with severe aortic stenosis for transfemoral transcatheter aortic valve implantation (TAVI) with a 23 mm or 27 mm LOTUS valve. Echocardiography, computed tomography, and electrocardiography data were evaluated by independent core labs. Post TAVI, 32.0% (72/225) of pacemaker-naïve patients underwent new PPM implantation at 30 days. Most (59/72, 82%) patients were implanted for third-degree atrioventricular block, and >10% overstretch of the LVOT by area was observed in 59.7% (43/72) of PPM patients. Significant independent predictors of PPM at 30 days included baseline RBBB (odds ratio [OR] 12.7, 95% CI: 4.5, 36.2; p<0.001) and LVOT overstretch >10% (OR 3.4, 95% CI: 1.7, 6.7; p<0.001). There was a trend towards a lower 30-day PPM rate in patients with a shallower (≤5 mm) implant depth (23.9% ≤5 mm vs. 36.9% >5 mm depth from LCS; p=0.06).
Conclusions: Careful attention to valve sizing and implant depth may help to reduce the rate of PPM with the LOTUS valve.
Abbreviations
AV: atrioventricular
CI: confidence interval
CT: computed tomography
ECG: electrocardiogram
LBBB: left bundle branch block
LVOT: left ventricular outflow tract
OR: odds ratio
PVL: paravalvular leak
RBBB: right bundle branch block
STS: Society of Thoracic Surgeons
TAVI: transcatheter aortic valve implantation
VARC: Valve Academic Research Consortium
Introduction
We have previously reported that, among 250 enrolled patients in the REPRISE II trial with extended cohort, 32.0% of pacemaker-naïve patients required a new PPM at 30 days, and 36.0% of pacemaker-naïve patients required a new PPM at one year. The objective of this analysis was to assess the incidence, timing, and predictors of the need for new PPM at 30 days in the REPRISE II trial with extended cohort.
Methods
STUDY DESIGN
The REPRISE II trial and LOTUS™ valve (Boston Scientific, Marlborough, MA, USA) have been described previously1 and are summarised here. In brief, the REPRISE II trial with extended cohort was a prospective, single-arm, international, multicentre study designed to evaluate the safety and effectiveness of the LOTUS valve. Per protocol, an initial cohort of 120 patients was enrolled, followed by an extended cohort of 130 patients giving a total of 250 patients enrolled under the same study design and protocol. The primary device performance endpoint was the 30-day mean aortic valve pressure gradient in the initial 120-patient cohort. The primary safety endpoint was 30-day all-cause mortality in the full cohort of 250 patients. Both primary endpoints were independently adjudicated and were compared with pre-specified performance goals of 18 mmHg and 16% mortality, respectively. Secondary endpoints were based on the Valve Academic Research Consortium (VARC)-2 guidelines. This study complied with the principles of the Declaration of Helsinki and all applicable local and country regulations. The ethics committee at each site approved the protocol prior to enrolment of the first patient and all patients or their legal guardians provided written informed consent. This study is registered at www.clinicaltrials.gov under the identifier NCT01627691.
DEVICE DESCRIPTION
The LOTUS™ Aortic Valve Replacement System (Boston Scientific) consists of a woven nitinol frame with three bovine pericardial leaflets. The valve is fully repositionable and retrievable, even after full expansion. The lower portion of the valve is coated with an Adaptive Seal™ (Boston Scientific) that minimises paravalvular leak (PVL). At the time the trial was conducted, only two valve sizes were available for clinical use, a 23 mm and a 27 mm prosthesis.
PATIENT SELECTION, PROCEDURE, AND FOLLOW-UP
Patients were considered eligible for enrolment if they were aged ≥70 years with severe symptomatic calcific aortic stenosis (New York Heart Association Class ≥II), and were at high or extreme risk for surgery, as defined either by a Society of Thoracic Surgeons (STS) score ≥8 or by Heart Team agreement of high surgical risk based on comorbidities and/or frailty. Patient eligibility was established by an internal Heart Team and confirmed prior to enrolment by an independent central case review committee.
All devices were implanted via the transfemoral route and balloon predilatation of the native aortic valve was required per protocol. All patients included in the trial had a preprocedural computed tomography (CT) angiogram for the purposes of valve sizing, including measurements of annulus and left ventricular outflow tract (LVOT) area and derived diameter, and overstretch of the annulus and LVOT (Figure 1).
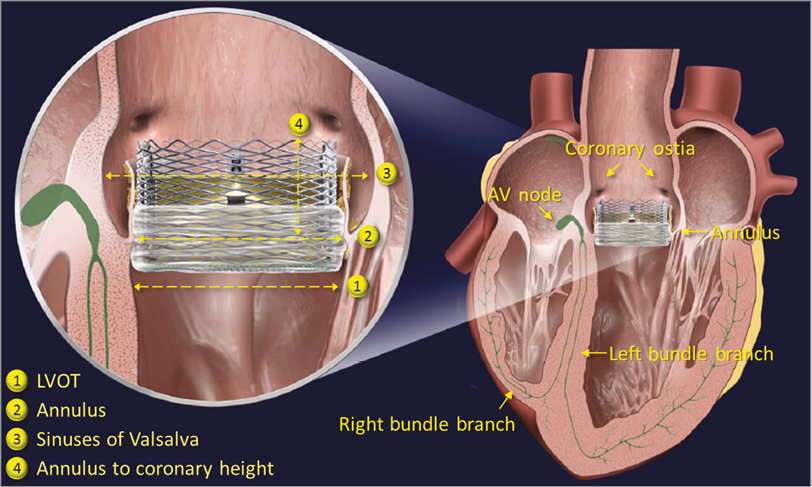
Figure 1. The LOTUS valve. Graphic of the LOTUS valve in situ.
The decision to implant and the timing of PPM implantation post TAVI were not predefined in the trial protocol but were left to the discretion of the participating sites per local standards.
STATISTICAL ANALYSIS
This analysis was performed on the as-treated population. Patients were included if they had sufficient clinical follow-up, or were known to have died or have had a PPM implantation within the analysis period, regardless of the length of available follow-up. Baseline, procedural characteristics, and outcomes were compared for patients with and without a newly implanted PPM up to 30 days and one year, and excluding 23 patients with a pre-existing pacemaker. Treatment groups were compared using a two-sided chi-square or Fisher’s exact test for categorical variables, as appropriate, and using the Student’s t-test for continuous variables. Clinical, anatomical, ECG, and procedural predictors (including baseline conduction disturbances, annular/LVOT size, overstretch and calcium; prosthesis size and implantation depth; gender; and order of enrolment at site) of the need for a newly implanted PPM at 30 days and one year were evaluated by multivariate analysis using logistic regression with Wald’s chi-square test; results are expressed as odds ratios with 95% confidence intervals. LVOT and annular overstretch were defined as the nominal valve area divided by the LVOT or annular area. All statistical analyses were performed using SAS software version 9.2 or above (SAS Institute, Inc., Cary, NC, USA).
Results
Among 250 enrolled patients, 23 patients had a pacemaker at baseline. Two patients did not have a LOTUS valve implanted during the index procedure; therefore, the as-treated 30-day analysis set comprised 248 patients (Figure 2). Of the two non-treated patients, one was not treated with a valve at baseline due to vascular complications but was later successfully implanted with a LOTUS valve on day 42. This patient is not included in the as-treated population at 30 days, but is included in the as-treated population at one year; therefore, the one-year analysis set comprised 249 patients. At 30 days, 32.0% (72/225) of pacemaker-naïve patients required a new PPM (Figure 2). Of these, 18 (25.0%) were implanted on day 0, 27 (37.5%) between day 1 and day 3, and the remainder between days 4 and 14. Between 31 days and one year, an additional nine patients required a new PPM, for a total PPM rate of 36.0% (81/225) in pacemaker-naïve patients at one year (Figure 2). Site-reported indications for PPM implant are shown in Table 1.
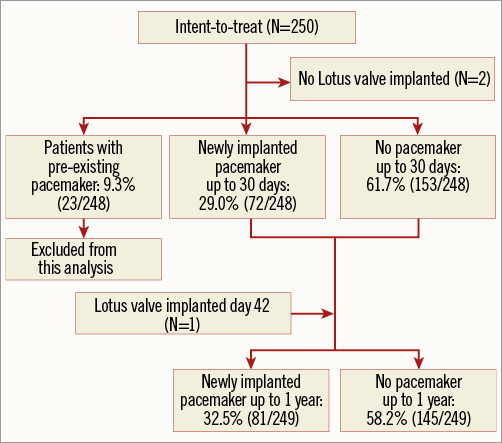
Figure 2. Patient flow chart. Disposition of patients in analysis.
CHARACTERISTICS OF PATIENTS WITH AND WITHOUT NEW PPM
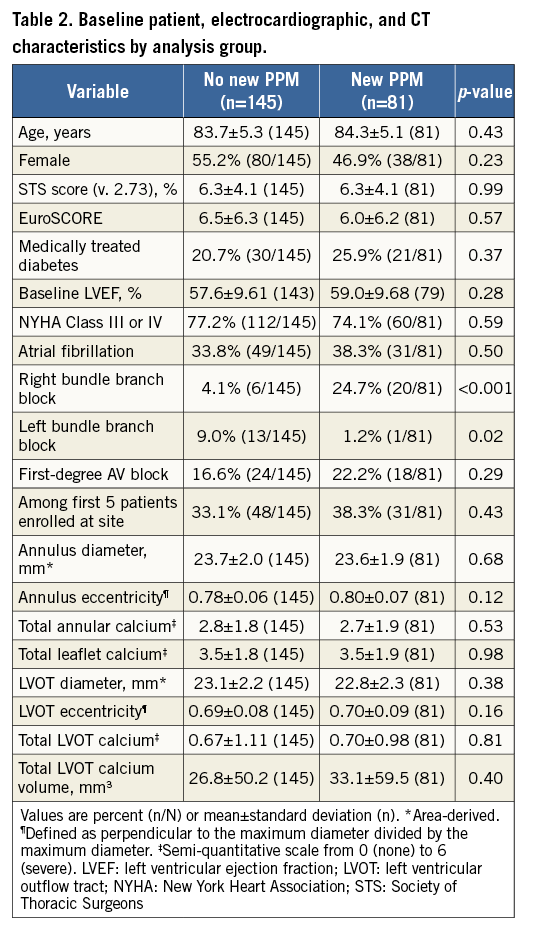
Patients who required a new PPM had a significantly higher incidence of right bundle branch block (RBBB) at baseline, whereas patients without a new PPM had a significantly higher baseline rate of left bundle branch block (LBBB) (Table 2). Baseline characteristics were otherwise similar between groups. Patients who were among the first five enrolled at each site were no more likely to require a new PPM than those enrolled later (p=0.43), suggesting that there was no learning curve associated with implanting the LOTUS valve. Baseline left ventricular ejection fraction (LVEF) did not differ between groups.
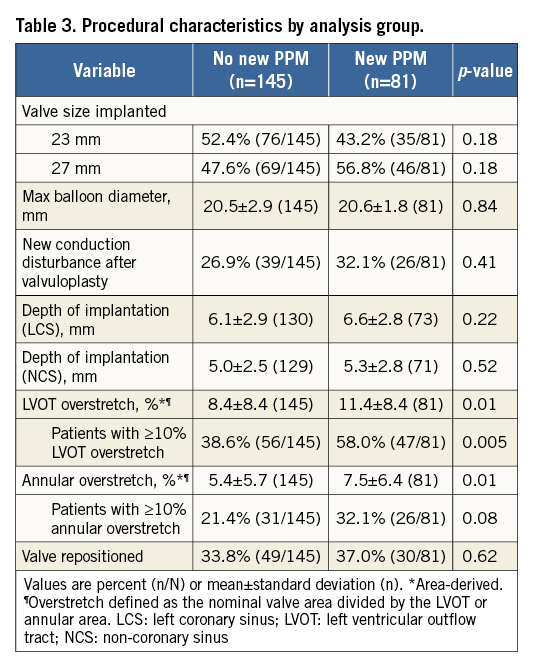
Procedural characteristics for patients with and without a new PPM are shown in Table 3. There was no significant effect of valve size or maximum predilatation balloon diameter on the need for a new PPM. Similarly, depth of implantation was not significantly different in this cohort between patients with and without a new PPM (Table 3). However, there was a trend towards a lower PPM rate in patients with a shallower (≤5 mm) implant depth (23.9% [16/67] ≤5 mm depth from left coronary sinus [LCS] vs. 36.9% [48/130] >5 mm depth from LCS; p=0.06, and 27.2% [28/103] ≤5 mm depth from non-coronary sinus [NCS] vs. 37.4% [34/91] >5 mm depth from NCS; p=0. 13). Overstretch of both the annulus and the LVOT was significantly greater in patients with a new PPM, and significantly more patients with PPM had >10% LVOT overstretch. Although a similar trend was present for >10% overstretch of the annulus, this did not reach statistical significance. Valve repositioning was not significantly associated with need for a new PPM.
CLINICAL OUTCOMES AT 30 DAYS AND ONE YEAR
All-cause mortality was not significantly different for patients with and without a new PPM at 30 days (3.9% no PPM vs. 5.6% new PPM; p=0.73), or at one year (12.6% no PPM vs. 12.3% new PPM; p=0.96). Similarly, LVEF was not different between groups at 30 days (54.8±10.0% no PPM vs. 53.2±6.8% new PPM; p=0.41), or at one year (53.4±10.4% no PPM vs. 50.9±9.0% new PPM; p=0.24). Among patients with a new PPM up to 30 days, 61.1% (33/54) were paced at 30 days and 55.4% (36/65) were paced at one year, as determined by the core laboratory review of ECGs. Systematic pacemaker interrogation was not required per protocol in the REPRISE II study and therefore rates of true pacemaker dependency could not be assessed.
PREDICTORS OF NEW PACEMAKER AT 30 DAYS
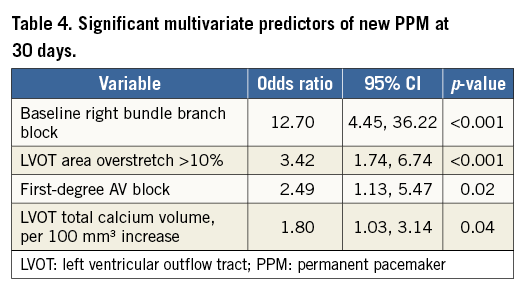
Significant predictors of new PPM in multivariate analysis at 30 days included baseline RBBB, LVOT overstretch >10% by area, first-degree AV block, and LVOT total calcium volume (Table 4). There were an insufficient number of new pacemakers between 31 days and one year to determine multivariate predictors of new PPM during that time frame.
Discussion
In the REPRISE II trial with extended cohort, 32.0% of pacemaker-naïve patients required a new PPM at 30 days, and an additional nine patients required a new PPM between 31 days and one year, giving a total PPM rate of 36.0% in pacemaker-naïve patients at one year. The most common indication for PPM implantation was third-degree AV block, particularly for implants within the first 30 days, and 63% of patients with new PPM were implanted within the first three days post procedure. Significant independent predictors of the need for new PPM at 30 days included baseline RBBB, LVOT overstretch >10% by area, first-degree AV block, and LVOT total calcium volume. There was no difference in mortality between patients with and without PPM at 30 days or at one year.
PATIENT-RELATED FACTORS
Consistent with multiple studies of other valves2-5, baseline RBBB and first-degree AV block were independent predictors of future need for new PPM with the LOTUS valve. The known mechanical stresses of the TAVI procedure, including balloon predilatation6, may have greater effect in patients with pre-existing conduction system disease, and more intensive post-procedural ECG monitoring of such patients may be warranted prior to hospital discharge. Similarly, calcification of the aorta and/or LVOT has been linked to increased need for new PPM with other valves7,8. This was also true in the current study, with LVOT total calcium volume emerging as a significant independent predictor of the need for a new PPM (OR 1.80 per 100 mm3 increase, 95% CI: 1.03, 3.14; p=0.04).
PROCEDURE-RELATED FACTORS
Overstretch, particularly of the LVOT, was significantly and strongly associated with an increased need for PPM with the LOTUS valve in this study. This is consistent with observations of other valves9-11. Overstretch, principally of the annulus, has been recommended with other transcatheter aortic valves to prevent the development of PVL. Other studies have demonstrated that there is an inverse relationship between PVL and PPM: as radial force exerted by the prosthetic valve increases – whether by increasing valve size or by the addition of a sealing skirt or cuff – the incidence of PVL decreases, but also the likelihood of conduction disturbances increases due to compression of the conduction system tissue4,12. Hence, there may be a trade-off in the choice of valve size between the potential development of PVL and the potential requirement for a new PPM. However, the LOTUS valve has consistently demonstrated very low rates of PVL, probably related to the Adaptive Seal, suggesting that oversizing is unnecessary with the LOTUS valve. The anticipated availability of an expanded LOTUS valve size matrix may help in this regard. It is also important to note that, in balancing the risk of PVL versus PPM, while moderate or greater PVL has been associated with increased mortality13-15, the majority of studies have found no association between mortality and the need for new PPM post TAVI6,16-18.
Unlike findings with the CoreValve® (Medtronic, Minneapolis, MN, USA)3,19, depth of implantation was not significantly associated with the need for a new PPM in this study, although there was a trend (p=0.06) towards a significantly lower PPM rate at 30 days in patients with a more shallow (≤5 mm) depth of implantation. Given this latter finding, it is possible that there is insufficient statistical power to assess the impact of depth of implantation in the current analysis. The ongoing RESPOND and REPRISE III studies may provide more information on this point.
COMPARISON WITH OTHER TRANSCATHETER VALVES
The 30-day rates of PPM following implantation with the LOTUS valve are higher than those observed with other second-generation valves, which have been reported in a range from 10% to 15% for the Edwards SAPIEN 3 valve (Edwards Lifesciences, Irvine, CA, USA)20-22 and 11% to 25% for the Medtronic CoreValve Evolut™ R valve23-26. Overstretch, final depth of implant, interaction between the prostheses and the LVOT and (potentially) other factors may all contribute to conduction disturbances following TAVI. There is variation in the LOTUS deployment technique, but the LOTUS frame frequently travels deeper into the LVOT during deployment than other TAVI systems. This may at least partially explain the observed difference in PPM implantation rates.
Study limitations
The REPRISE II trial with extended cohort is a single-arm study with no active control, and lacks statistical power to determine differences for some potential baseline predictors as well as mortality outcomes; however, to date, the study represents the largest published cohort of patients with follow-up to one year with the LOTUS valve. Secondly, the indications for PPM implant were site-reported and not centrally adjudicated, which could have resulted in inter-site variability in reporting of indications. Third, in this study, only two valve sizes were available, which may have contributed to overstretch of the valve. Fourth, the indications and timing of PPM implantation post TAVI were not predefined in the trial protocol but were left to the discretion of the participating sites per local standards, which resulted in a wide variation between sites in both post-TAVI PPM rates and indications for PPM implantation. Finally, the study did not include a provision for systematic interrogation of PPM in the study protocol, so assessment of true pacemaker dependency was not possible. This topic will be further evaluated in the ongoing REPRISE III trial, which has included a pacemaker interrogation algorithm as part of the protocol-mandated data collection.
Conclusions
In summary, the results of these analyses suggest that appropriate valve sizing that avoids overstretch, particularly of the LVOT, and implanting the valve higher (≤5 mm where possible without compromise of the coronary ostia) may be helpful in reducing the need for PPM post procedure with the LOTUS valve. Additionally, although the majority of PPM implants were for clearly appropriate indications, a minority of devices were implanted in patients for less well-established indications such as LBBB and first-degree AV block. Physicians should follow recommended treatment guidelines for the implantation of a new PPM to ensure optimal patient management post TAVI.
| Impact on daily practice In this prospective study of 250 patients treated with the LOTUS valve, 32.0% of pacemaker-naïve patients underwent new PPM implantation at 30 days and 36.0% at one year. Of these, 62.5% were implanted by day 3 post procedure and 55.4% were paced at one year by ECG analysis. Baseline RBBB and LVOT overstretch were significant independent predictors of the need for PPM at 30 days following LOTUS valve implantation. Careful attention to valve sizing and positioning may help to reduce the rate of PPM with the LOTUS valve. |
Acknowledgements
The authors thank Hong Wang, MS (Boston Scientific Corporation), for statistical analysis.
Funding
The REPRISE II study with extended cohort was funded and sponsored by Boston Scientific Corporation (Marlborough, MA, USA).
Conflict of interest statement
N. Dumonteil has received consultant fees/honoraria from Boston Scientific, Edwards, and Medtronic, and proctor fees from Boston Scientific, Edwards, and Medtronic. I. Meredith has received grant support/research contracts from Boston Scientific, and Medtronic, and consultant fees/honoraria from Boston Scientific. D. Blackman has received consultant fees/honoraria from Boston Scientific and Medtronic. D. Hildick-Smith has received proctor/consultant fees from Medtronic, Edwards, and Boston Scientific. M. Spence has received honoraria/proctor fees from Boston Scientific, Edwards, and Medtronic. D. Walters has received grant support/research contracts from Edwards, Boston Scientific, and St. Jude, and consultant/proctor fees from Edwards and Boston Scientific. J. Harnek has received proctor fees from Boston Scientific. S. Worthley has received grant support/research contracts from Medtronic, St. Jude Medical, and Boston Scientific, and honoraria from Medtronic, and St. Jude Medical. G. Rioufol has received consultant fees/honoraria from Medtronic, and St. Jude, and grant support from Hexacath. T. Lefèvre has received consultant fees/honoraria from Boston Scientific, Edwards, Sanofi, and Tryton, and grant support/research contracts from Boston Scientific, Direct Flow, Symetis, and The Medicines Company. N. Van Mieghem has received research grants from Boston Scientific. V. Houle, D. Allocco and K. Dawkins have salary/equity interest in Boston Scientific. D. Tchétché and T. Modine have no conflicts of interest to declare.

