
Abstract
Aims: We sought to evaluate the impact of permanent pacemaker (PPM) implantation on two-year mortality and one-year left ventricular ejection fraction recovery (∆LVEFR=one-year LVEF–baseline LVEF) after transcatheter aortic valve implantation (TAVI).
Methods and results: We pooled patient-level data from four European institutions with significant TAVI volume. Outcomes were compared between patients without PPM (no-PPM), patients with PPM prior to TAVI (old-PPM) and patients with PPM implanted after TAVI (new-PPM). Out of 1,062 patients included in the pooled data set, 783 (73.7%) were in the no-PPM group, 164 (15.4%) in the new-PPM group and 115 (10.8%) in the old-PPM group. All-cause and cardiovascular mortality at two years were similar for patients with no-PPM and new-PPM (adjusted HR 1.11, 95% CI: 0.74-1.67; p=0.62; and adjusted HR 1.16, 95% CI: 0.68-1.98; p=0.59). Conversely, old-PPM was associated with increased risk of both all-cause and cardiovascular mortality vs. no-PPM. By multivariable analysis new-PPM did not affect LVEFR, while old-PPM did. We observed a multiplicative interaction, between new-PPM and post-procedural aortic regurgitation ≥1+ on two-year mortality and one-year LVEFR, with increased risk of death and impaired LVEFR in patients with new-PPM and post-procedural aortic regurgitation (PPAR) ≥1+ (both pinteraction<0.0001).
Conclusions: In patients undergoing TAVI, the presence of a PPM at baseline yielded a negative effect on long-term prognosis while new-PPM did not. The combination of new-PPM with PPAR adversely impacts on survival and LV function recovery.
Introduction
With over 200,000 procedures performed worldwide, transcatheter aortic valve implantation (TAVI) has become the treatment of choice for patients affected by severe aortic stenosis (AS) deemed at prohibitive or high risk for surgical aortic valve replacement (SAVR)1. In order for TAVI to expand to intermediate and even low surgical risk patients, several relatively common procedural complications need to be reduced. Atrioventricular conduction disturbances requiring permanent pacemaker (PPM) implantation are fairly common post TAVI, ranging between 5% and 12% with the balloon-expandable Edwards SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA) and even more than 30% with use of the self-expanding Medtronic CoreValve ReValving® System (Medtronic, Minneapolis, MN, USA)2,3. While clinical, anatomical and procedural factors associated with higher risk of PPM implantation after TAVI have been identified, the clinical significance and long-term impact on mortality of this complication are still controversial4. Strong evidence supports the negative effect of permanent right ventricular apical pacing on cardiac physiology and long-term outcomes, which is associated with higher risk of mortality and rehospitalisation for heart failure5-8. Previous studies have suggested that PPM implantation after TAVI is a relatively benign complication, not associated with higher risk of midterm and long-term mortality9,10. However, the need for PPM implantation post TAVI seems to be associated with impaired left ventricular ejection fraction recovery (LVEFR) compared with patients who do not require a PPM9. In the light of the controversies surrounding this issue, and its potential clinical implications, we sought to investigate the effect of PPM implantation on mortality and LVEFR after TAVI from the large, multicentre, PRAGMATIC (Pooled Rotterdam-Milan-Toulouse in Collaboration) registry. In detail, the objectives of the present study were: (i) to evaluate the unadjusted and adjusted impact of PPM on long-term all-cause and cardiovascular mortality after TAVI; (ii) to estimate the predictors of new-PPM implantation after TAVI; and (iii) to investigate the effect of PPM on LVEFR at one year after the index procedure.
Methods
STUDY POPULATION AND DESIGN
The PRAGMATIC initiative is a collaboration among four European institutes with significant TAVI volume. Baseline and procedural characteristics and clinical outcomes were prospectively collected from the start of the TAVI programme in the respective centres, from November 2005 to December 2011. Data were subsequently pooled in a structured data set with pre-specified data fields. Institutions involved in the PRAGMATIC initiative were San Raffaele Scientific Institute (Milan, Italy), Clinique Pasteur (Toulouse, France), Thoraxcenter, Erasmus Medical Center (Rotterdam, The Netherlands), and Rangueil University Hospital (Toulouse, France). Follow-up was performed as per local clinical practice by means of phone calls or in-person visits. No independent clinical event adjudication of adverse events was performed.
For the purpose of the present study, the patient population was categorised into three groups: (i) patients with no PPM before or after the procedure (no-PPM group); (ii) patients with PPM at baseline prior to the procedure (old-PPM group); and (iii) patients with the need for PPM implantation after the procedure (new-PPM group). The present study was approved by an institutional review board and the included subjects gave informed consent for data collection and analyses.
TAVI PROCEDURE
Patients included in this analysis underwent TAVI procedure through various available access modalities including percutaneous transfemoral, surgical transfemoral, transapical, transaxillary, and transaortic access. The TAVI devices used in the procedure were the SAPIEN and SAPIEN XT valves (both Edwards Lifesciences) and the CoreValve® (Medtronic). Valve type and size choice were at the discretion of the operator following multimodality imaging evaluation of patients’ anatomy.
INDICATION FOR PERMANENT PACEMAKER IMPLANTATION
Patients with new-onset conduction disturbances were evaluated by an electrophysiologist in each centre. Permanent pacemaker implantation after TAVI was indicated in case of high-degree atrioventricular block (third-degree or advanced second-degree atrioventricular block) which was not expected to resolve, or in the presence of sinus node dysfunction and documented symptomatic bradycardia in agreement with American guidelines11. The decision whether to implant a PPM in case of left bundle branch block with PR prolongation (>200 milliseconds), without signs of normalisation, was left to the electrophysiologist’s discretion.
Study endpoints
The co-primary endpoints of this study were all-cause and cardiovascular mortality. All-cause and cardiovascular mortality were reported at 30 days, one year and two years. LVEFR was defined as the difference between the measured LVEF at one year and LVEF at baseline (∆LVEFR=one-year LVEF–baseline LVEF). LVEF was measured with the Simpson’s biplane method. Clinical endpoints in our study were collected and defined according to the Valve Academic Research Consortium criteria.
STATISTICAL ANALYSIS
Values were expressed as mean±standard deviation, median (Q1 and Q3 values) or percentages, as appropriate. Variables were tested for normality using the Kolmogorov-Smirnov test. Group differences were tested using the Student’s t-test or Wilcoxon signed-rank test for normally and non-normally distributed continuous variables, respectively. The chi-square test or Fisher’s exact test was used, as appropriate, to test group differences of proportions. Crude all-cause and cardiovascular mortality rates were calculated using the Kaplan-Meier method. Comparisons between groups were performed using the log-rank test. Cross-sectional multivariable logistic regression analysis was performed to identify factors associated with new PPM implantation. Longitudinal multivariable Cox regression analysis was used to estimate the effect of PPM status on all-cause and cardiovascular mortality. Multivariable linear regression was used to evaluate the independent effect of PPM on total days of hospitalisation and LVEFR at one year. Multivariable logistic, Cox and linear regression modelling was performed through a backward stepwise process with covariate entry and exit thresholds set to 0.05 and 0.1, respectively. Candidate variables for inclusion were chosen based on previously identified risk factors for mortality, with the total number of variables limited to avoid model overfitting according to the number of events (10:1 ratio). Across-centre heterogeneity was accounted for in all the multivariable models by using “centre” identifiers as a stratification variable. Statistical analysis was performed using SPSS software, Version 21 (IBM Corp., Armonk, NY, USA). Additional statistical methods are described in the Online Appendix.
Results
BASELINE CHARACTERISTICS
Out of 1,062 patients included in the pooled data set, 783 (73.7%) were in the no-PPM group, 164 (15.4%) in the new-PPM and 115 (10.8%) in the old-PPM group. Median follow-up time in the overall cohort was 425 days (interquartile range 255 to 712 days), with no significant differences among groups. A total of 163 and 358 patients were censored at follow-up at one and two years, respectively. Baseline clinical and procedural characteristics are summarised in Table 1. Patients in the old-PPM group were more commonly male and had a higher prevalence of chronic kidney disease, peripheral vascular disease and a higher logistic EuroSCORE. Moreover, compared with patients with no and new PPM, those in the old-PPM group had a lower left ventricular ejection fraction and aortic mean and peak gradient. Patients with new PPM were more commonly treated with the CoreValve, had a higher cover index and more commonly required balloon post-dilation. There were no differences in arterial access site among groups. Procedural characteristics across groups are described in Online Table 1.
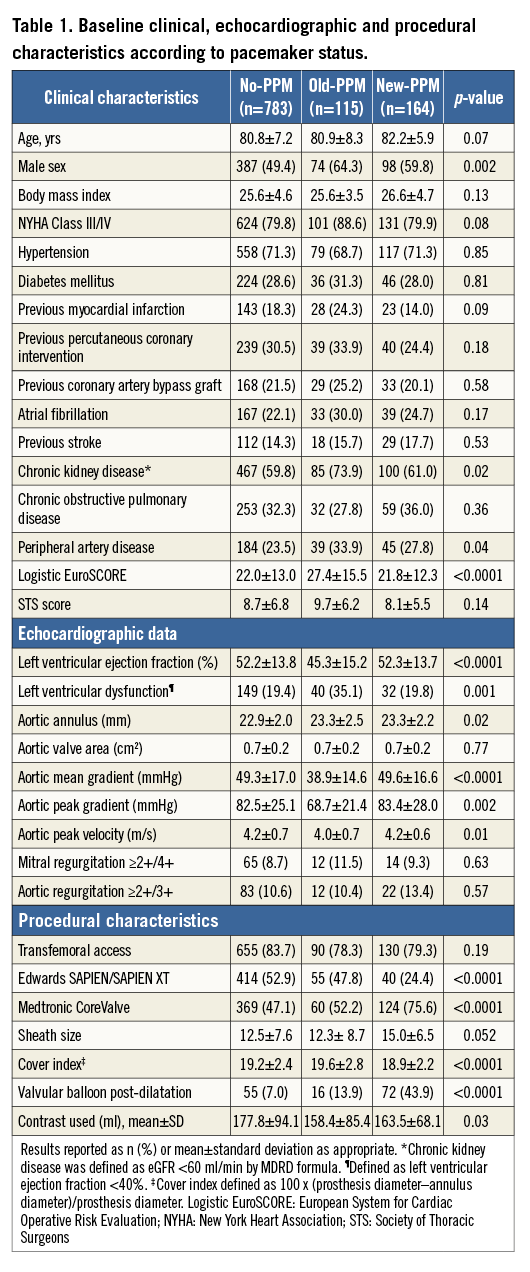
PREDICTORS OF NEW PERMANENT PACEMAKER IMPLANTATION
Predictors of new PPM after TAVI are reported in Figure 1. Age (per year increase), male gender, body mass index (per unit increase), CoreValve use, valvular balloon post-dilation after valve implantation and cover index (>8 units) independently predicted new PPM implantation. Conversely, femoral access use was independently associated with a lower risk of new PPM requirement after TAVI.
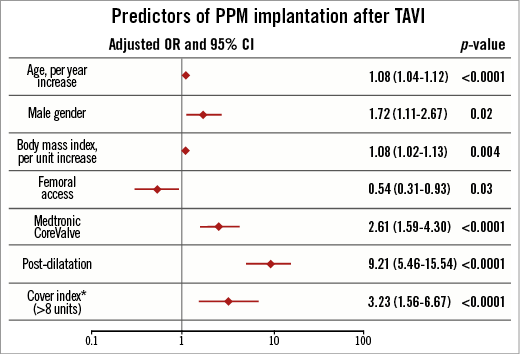
Figure 1. Predictors of PPM after TAVR. *Cover index defined as 100 x (prosthesis diameter–annulus diameter)/prosthesis diameter. OR: odds ratio
THIRTY-DAY AND TWO-YEAR CLINICAL OUTCOMES
There were no significant differences in device success and post-procedural aortic regurgitation (PPAR) among groups (Online Table 2). Conversely, compared with no-PPM and old-PPM, patients in the new-PPM group had a longer in-hospital stay (9.5±7.7 vs. 9.9±9.2 vs. 12.2±8.0 days; p<0.0001). This association persisted following multivariable adjustment (adjusted beta coefficient: +3.0; 95% CI: 1.6-4.5; p<0.0001).
Kaplan-Meier and Cox regression estimates for all-cause and cardiovascular mortality are reported in Table 2, Figure 2A, Figure 2B and Online Figure 1. Compared with no-PPM, old-PPM was associated with higher unadjusted and adjusted risk of 30-day and two-year all-cause and cardiovascular mortality. No differences were observed in 30-day mortality risk between no-PPM and new-PPM. Conversely, while a trend towards higher crude all-cause and cardiovascular two-year mortality was observed between no-PPM and new-PPM, this association was lost following multivariable adjustment with substantial attenuation of the effect for both endpoints.
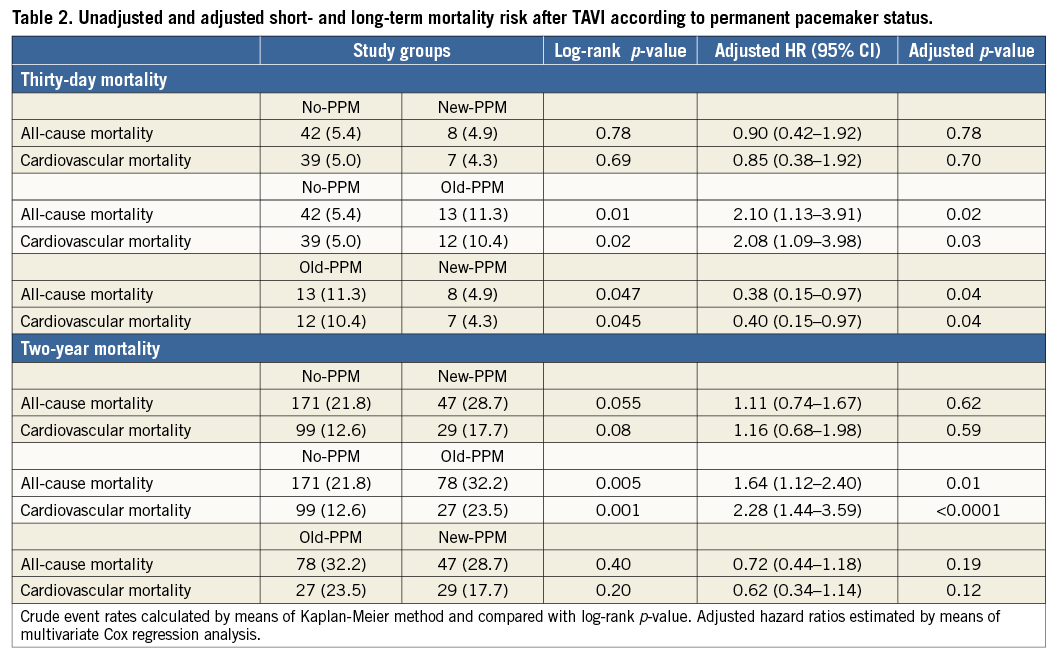
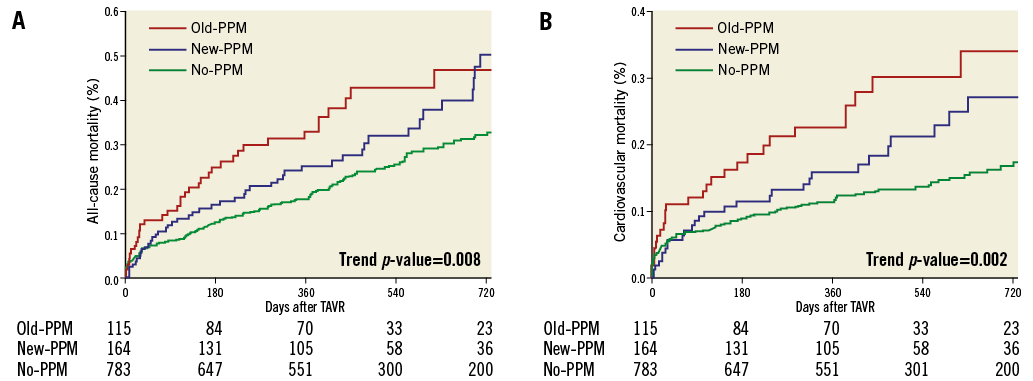
Figure 2. Kaplan-Meier curves. A) All-cause mortality according to PPM status after TAVI. B) Cardiovascular mortality according to PPM status after TAVI. PPM: permanent pacemaker
Subgroup analyses for two-year all-cause mortality in patients with new and old PPM are reported in Figure 3A and Figure 3B, respectively. A significant multiplicative effect was observed between PPAR ≥1+ and new-PPM implantation (Figure 4A) with higher risk of mortality in patients with PPAR and new PPM (adjusted HR 2.34, 95% CI: 1.48-3.71) compared to patients without PPAR and new PPM (adjusted HR 0.87, 95% CI: 0.50-1.51; pinteraction <0.0001). By subgroup analysis in patients with old PPM, a significant heterogeneity in the magnitude and directionality of the effect was observed in patients with a clinical history of MI (Figure 4B), with higher mortality risk associated with old PPM in those without prior MI (adjusted HR 2.31, 95% CI: 1.55-3.44) and lower in those with prior MI (adjusted HR 0.48; 95% CI: 0.17-1.40; pinteraction<0.0001).
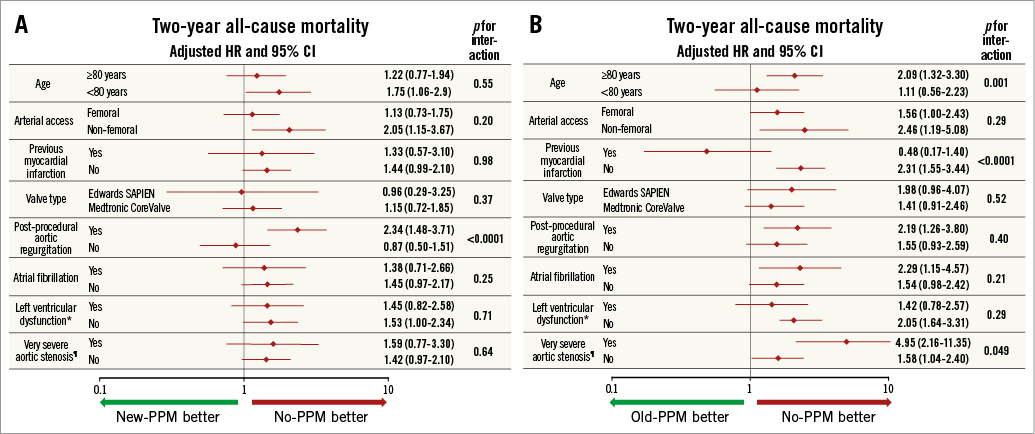
Figure 3. Subgroup analysis for two-year all-cause mortality. A) Between new-PPM and no-PPM. B) Between old-PPM and no-PPM. *Defined as left ventricular ejection fraction <40%. ¶Defined as aortic stenosis with mean gradient >60 mmHg.
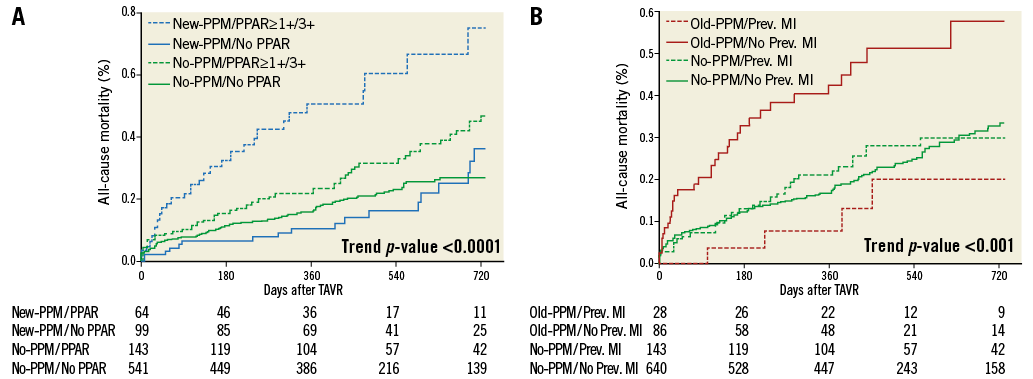
Figure 4. Kaplan-Meier curves for all-cause mortality. According to new-PPM versus no-PPM and post-procedural aortic regurgitation (A) and old-PPM versus no-PPM and previous myocardial infarction (B).
EFFECT OF PERMANENT PACEMAKER ON LEFT VENTRICULAR EJECTION FRACTION RECOVERY
Baseline LVEF, one-year LVEF and LVEFR in patients for whom one-year echocardiographic follow-up was available (N=422) are reported in Figure 5. Significant changes in LVEF were observed in all the PPM subgroups, with the highest positive change observed in those with no PPM (∆+3.7%), a lower positive change in LVEF in those with old PPM (∆+0.7%) and a negative change in LVEF observed in those with new PPM (∆–2.3%; p<0.0001). Predictors of LVEFR are reported in Table 3. Following multivariable adjustment, new PPM had no effect on LVEFR. Conversely, while old PPM had no effect on LVEFR at univariable analysis, it became independently and inversely associated with LVEFR after multivariable adjustment. Importantly, the effect of new PPM on impaired LV recovery was significantly influenced by the presence of residual PPAR, with higher risk of impaired LV recovery in those with new PPM and PPAR ≥1+ (OR 8.48, 95% CI: 1.99-36.12), and lower risk in those with new PPM and no PPAR (OR 0.57, 95% CI: 0.23-1.39; pinteraction=0.002).
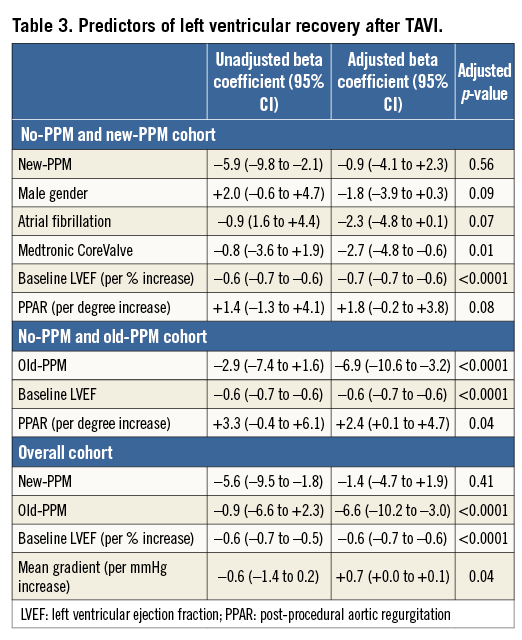
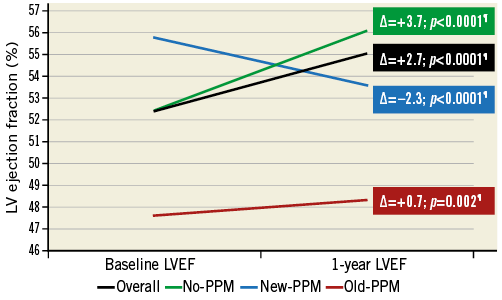
Figure 5. Left ventricular ejection fraction at baseline and one year according to PPM status. ¶p-value calculated with paired t-test. LVEF: left ventricular ejection fraction; PPM: permanent pacemaker
EFFECT OF PERMANENT PACEMAKER ON CLINICAL STATUS
New York Heart Association (NYHA) class among groups at baseline, one and two years is reported in Figure 6. At baseline, patients in the old-PPM group had a significantly worse NYHA status. A significant improvement from baseline to one year was observed in all groups (p<0.0001). Finally, no differences in NYHA class were observed across PPM groups at both one and two years.
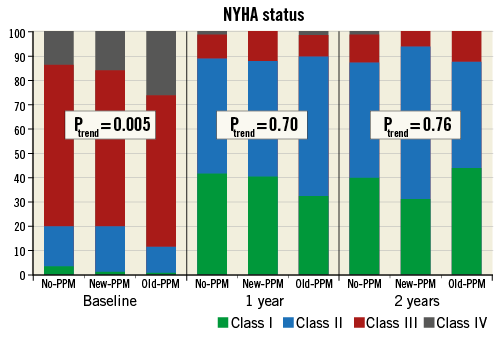
Figure 6. New York Heart Association class at baseline, one year and two years according to pacemaker status. NYHA: New York Heart Association; PPM: permanent pacemaker
Discussion
The main findings of the PRAGMATIC Pacemaker substudy are: (i) the presence of PPM at baseline was associated with a higher clinical risk profile and a higher risk of mortality at 30 days and two years of follow-up; (ii) several clinical and procedural factors independently predicted the need for PPM after TAVI, including anti-PPAR strategies such as valvular post-dilation and device oversizing; (iii) in the overall population, new-PPM implantation had no effect on either long-term all-cause or cardiovascular mortality after TAVI; however, it was associated with longer in-hospital stay; (iv) new PPM did not independently correlate with lower LVEFR, while old PPM did; (v) we observed a multiplicative interaction between new PPM and PPAR ≥1+ on the risk of two-year mortality and impaired LV recovery at one year.
Conduction abnormalities with the need to implant a PPM are one of the most common complications after TAVI2. The pathophysiological substrate underlying this complication is due to the spatial proximity between the aortic valve complex and the pathways of the atrioventricular conduction system3. This topographic relationship may lead to a mechanical interaction between the prosthesis frame and the conduction system with subsequent inflammation and ischaemic injury of the conduction pathways resulting in high-grade or complete atrioventricular block3. Alongside new-onset conduction disturbances, PPAR represents another important complication of TAVI which has been consistently associated with increased late morbidity and mortality in previous studies1. However, while new-generation TAVI devices have been specifically designed to mitigate the risk of PPAR after TAVI through different anti-PPAR technological properties, the need for post-TAVI PPM implantation remains substantial12.
IMPACT OF BASELINE PERMANENT PACEMAKER ON MORTALITY
PPM at baseline was consistently associated with a higher risk of early and late mortality. While this association might reflect the result of confounding bias despite multivariable adjustment, as patients with baseline PPM were older and had higher clinical risk profile, the simple presence of PPM at baseline might be taken into account for optimal risk stratification of patients with severe AS. However, old PPM might also have a direct role in causing mortality, as long-term pacing is known to be associated with a higher risk of heart failure, especially in patients with left ventricular dysfunction5-8. Of note, the deleterious effect of old PPM did not seem to apply to patients with a history of MI, in whom old PPM had a protective effect, confirming results from randomised controlled trials and current guidelines11.
PREDICTORS OF PERMANENT PACEMAKER IMPLANTATION AFTER TAVI
Several clinical and procedural factors predicted the need for PPM implantation after TAVI. We observed a significant association between new PPM and valvular balloon post-dilation and device oversizing. While these are commonly implemented anti-PPAR procedural strategies, they might increase the risk of conduction disturbances through more traumatic injury of the conduction pathways.
LACK OF IMPACT OF NEW PERMANENT PACEMAKER ON MORTALITY
The need for new PPM was not associated with an increase in late mortality, which corroborates prior large studies which have investigated this issue9,10. However, a recent report from the PARTNER trial showed higher crude rates of repeat hospitalisation or all-cause mortality and, as in our study, a trend towards higher all-cause mortality with new PPM13. Despite this study having the strength of including a large patient population and prospectively collected high-quality data, the analyses were not adjusted for baseline clinical variables and thus subject to significant confounder bias. In concert with our observation, it is likely that the need for PPM after TAVI most commonly occurs in sicker patients, thereby explaining the observed substantial attenuation of the effect of PPM on mortality following multivariable adjustment. It might be expected that with the expansion of TAVI towards lower-risk patients the occurrence of this complication might be less common as a result of a lower prevalence of pathologic substrates predisposing to irreversible conduction pathway injury. We did not capture PPM dependence during follow-up. It is plausible that patients with a PM prior to TAVI were more PPM dependent than the patients who required a PPM after TAVI. In fact, it is well known that approximately 50% of patients are no longer PM dependent within weeks following the procedure, suggesting a healing of the conduction system over time14.
IMPACT OF NEW PERMANENT PACEMAKER IN PATIENTS WITH POST-PROCEDURAL AORTIC REGURGITATION
Similar to what was reported by Urena et al9, we did not find a significant interaction between PPM and valve type, and left ventricular dysfunction. Instead, a significant interaction was observed between PPAR ≥1+ and new PPM on the risk of mortality and of impaired LVEFR. Several potential mechanisms may explain this differential effect. First, concomitant new-onset PPAR and new PPM might synergistically affect left ventricular haemodynamics, with subsequent accelerated adverse LV remodelling and development of heart failure. Second, the presence of a PPM might abrogate the LV adaptation (Frank-Starling’s mechanism) to the PPAR-related pre-load increase15, implying an LV volumetric overload and retrograde congestion. However, irrespective of the mechanism, the interaction between these two procedural complications might have important clinical implications on long-term TAVI outcomes. Considering that implementation of anti-PPAR strategies increases the risk of new-PPM implantation, the counterbalancing influences between preventing residual PPAR through oversizing or post-dilating the implanted valve while increasing the risk of PPM have to be taken into account when TAVI is planned or performed. Moreover, optimal device implantation, especially with CoreValve, is critical in preventing both types of complication, as it has been demonstrated that lower implantation depth is significantly associated with higher risk of residual PPAR and new-onset conduction disturbances with need of PPM implantation16.
EFFECT OF PERMANENT PACEMAKER ON LEFT VENTRICULAR FUNCTION
Conversely to patients with old PPM and no PPM, those requiring new PPM experienced a significant crude reduction in LVEF at one year, therefore with negative LVEFR. However, following multivariable adjustment, new PPM was not independently associated with a worsening in LVEF at linear regression analysis. In contrast, old PPM was independently associated with a worsening in LVEF, suggesting that substantial confounding factors affect interpretation of crude clinical and functional TAVI data. This observation, which is discordant with that reported by Urena et al9, might be the result of a different PPM implantation selection and/or discrepancies in the multivariable adjustment models. The lack of a detrimental effect on LV function of new PPM is reassuring and fits with the absence of an increase in mortality observed at clinical follow-up.
Limitations
Our study has several important limitations that need to be disclosed. First, this is a retrospective post hoc analysis from a prospective real-world registry; therefore, our results have to be considered hypothesis-generating. Second, despite multivariable adjustment, our effect estimates are subject to potential residual confounder bias. Third, important data such as baseline conduction disturbances, indication to PPM implantation, type of PPM implanted and PPM dependency over time were not available in the pooled data set. Fourth, the present results may be subject to bias related to device selection during the setting up of the TAVI programmes at the different sites and indications for new-PPM implantation across centres. Fifth, new-generation TAVI devices, which have been associated with improved performances17, are not included in this registry, thereby limiting the current external validity of our findings. Sixth, echocardiographic follow-up at one year was available in less than 50% of patients, introducing possible selection bias. Finally, although we did account for inter-centre heterogeneity by including centre identifiers in our multivariable models, some degree of heterogeneity on the effect estimates may persist.
Conclusions
In patients undergoing TAVI, the presence of a PPM prior to the procedure appears to be associated with an increased risk of short- and long-term mortality. Conversely, new-PPM implantation prolongs in-hospital stay, but, overall, has no effect on mortality up to two years of follow-up. New PPM was associated with a significantly increased risk of all-cause mortality and impaired LVEFR in patients who develop PPAR. Considering that anti-PPAR strategies (i.e., post-dilation or oversizing) increase the risk of new PPM implantation, their benefits and risk must be taken into account when TAVI is performed. The results of the present study further characterise the clinical significance of PPM in patients undergoing TAVI and elucidate its effect in relevant subgroups of patients.
| Impact on daily practice The presence of PPM at baseline identifies a high-risk subset of patients undergoing TAVI. While new PPM implantation after TAVI did not seem overall to be associated with adverse prognosis and worse left ventricular function, the concomitance of residual PPAR and PPM appeared to be associated with an increased risk of mortality and impaired LVEFR. Post-dilation and oversizing were associated with an increased likelihood of the need for new PPM. TAVI operators should carefully balance anti-PPAR strategies with the risk of new-onset conduction disturbances and the need for chronic pacing. This study critically underscores the importance of optimal device implantation techniques in determining long-term TAVI outcomes. |
Conflict of interest statement
D. Tchetche, N. Dumonteil, and B. Marcheix are proctors for Edwards Lifesciences and Medtronic. P. de Jaegere is a proctor for Medtronic. N. Dumonteil is also a proctor for Boston Scientific and a consultant for Biotronik. The other authors have no conflicts of interest to declare.
SUPPLEMENTARY DATA
Online Appendix
STATISTICAL METHODS
Multivariable logistic, Cox and linear regression modelling was performed through a backward stepwise process with covariate entry and exit thresholds set to 0.05 and 0.1, respectively. Candidate variables for inclusion were chosen based on previously identified risk factors for mortality, with the total number of variables limited to avoid model overfitting according to the number of events (10:1 ratio). Candidate variables for the multivariable (Cox regression, logistic regression and linear regression) models included: age, gender, body mass index, peripheral vascular disease, chronic obstructive pulmonary disease, diabetes mellitus, chronic kidney disease, prior myocardial infarction (MI), baseline mitral regurgitation, baseline aortic regurgitation, baseline LVEF, very severe AS (mean transvalvular gradient >60 mmHg), procedural access site, valvular balloon post-dilation, in-hospital cerebrovascular events, in-hospital life-threatening bleeding, in-hospital acute kidney injury stage 2 or 3, in-hospital major vascular complications and post-procedural aortic regurgitation (PPAR). Across-centre heterogeneity was accounted for in all the multivariable models by including “centre” identifiers as a stratification variable.
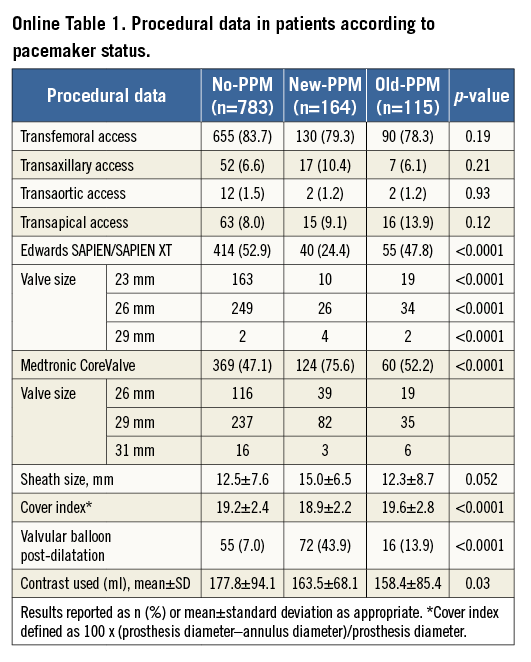
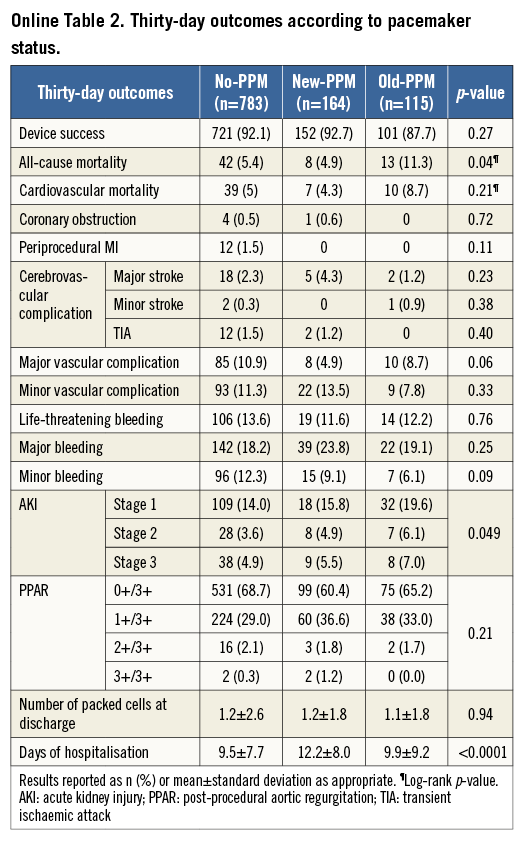
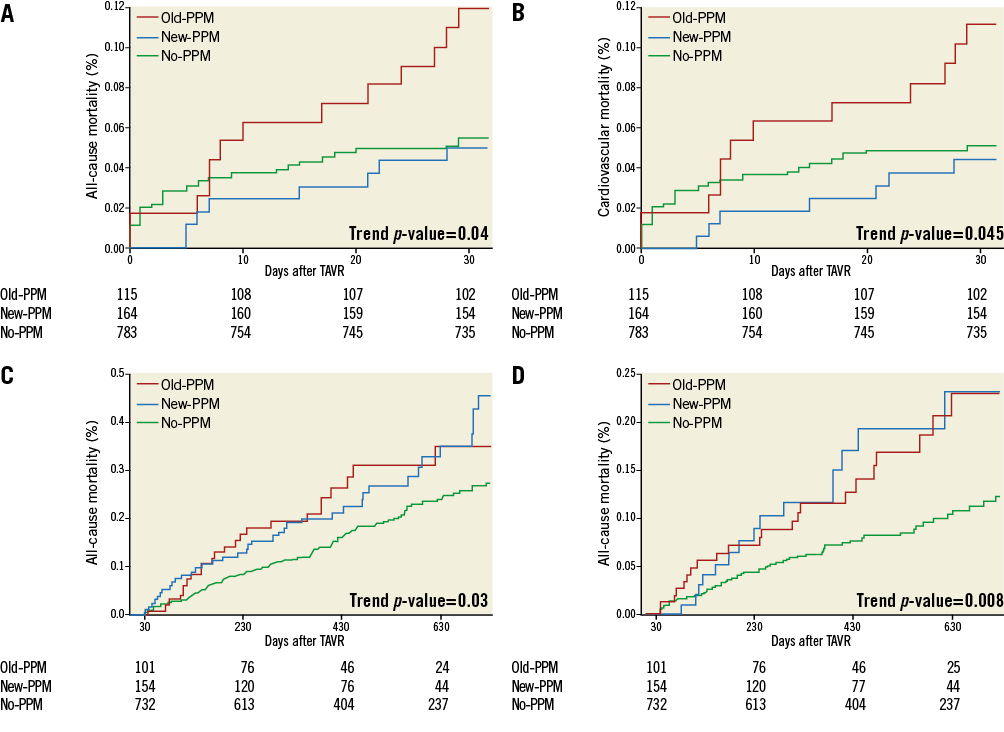
Online Figure 1. Kaplan-Meier curves for early (30 days) all-cause (A) and cardiovascular mortality (B), and late (>30 days) all-cause (C) and cardiovascular mortality (D) according to permanent pacemaker status. PPM: permanent pacemaker

