
Introduction
Among patients with severe calcific aortic valve stenosis who are at high risk for surgical intervention, transcatheter aortic valve replacement (TAVR) is a safe and effective treatment option1,2. The REPRISE I feasibility study3 was designed to assess the acute safety and performance of TAVR with the mechanically expandable, fully repositionable and retrievable LOTUS™ valve (Boston Scientific, Marlborough, MA, USA). In addition to controlled mechanical expansion, which facilitates precise positioning, the valve has a unique adaptive seal, resulting in minimal paravalvular aortic regurgitation. This report of five-year outcomes from the REPRISE I study represents the longest-term data available to date for the LOTUS valve.
Methods
REPRISE I was a prospective, single-arm study of the first-generation LOTUS valve system in 11 female patients with severe, symptomatic aortic valve stenosis. Patients could be enrolled in the study if they met at least one of the following criteria: Society of Thoracic Surgeons (STS) score ≥8% or a EuroSCORE ≥20% or documented multidisciplinary Heart Team agreement that the patient was at high risk for surgery due to frailty and/or coexisting comorbidities. The clinical study design and primary endpoint have been described previously3. Echocardiographic measurements, including valve performance and cardiac function, were performed during clinical follow-up at hospital discharge, 30 days post TAVR, and annually up to five years. An independent clinical events committee adjudicated death, myocardial infarction, neurologic events, and other Valve Academic Research Consortium (VARC)-defined events4.
Results
Baseline patient characteristics, echocardiographic assessments, and procedural characteristics were as described previously3. Briefly, the mean age of the patients was 83.0±3.6 years, the mean STS score was 4.9±2.5%, and the mean logistic EuroSCORE was 9.5±4.4%.
All patients were successfully implanted with a LOTUS valve, with no procedural mortality. The survival rate was 100% up to two years, 90.9% at three years, 72.7% at four years, and 63.6% at five years (4/11 patients died, including cardiovascular death in one patient). Of the seven surviving patients, six patients (85.7%) had five-year clinical follow-up available (Table 1). The cumulative major stroke rate at five years was 9.1% (one patient experienced a major stroke on day two). Conduction disturbance requiring new permanent pacemaker implantation occurred in four patients within the first 30 days, with none subsequently. There were no hospitalisations for valve-related symptoms or cardiac decompensation; however, two patients experienced periprocedural vascular complications (major in one patient; minor in one patient), and life-threatening/disabling bleeding occurred in two patients (on day 14 in one patient, and on day 20 in one patient).
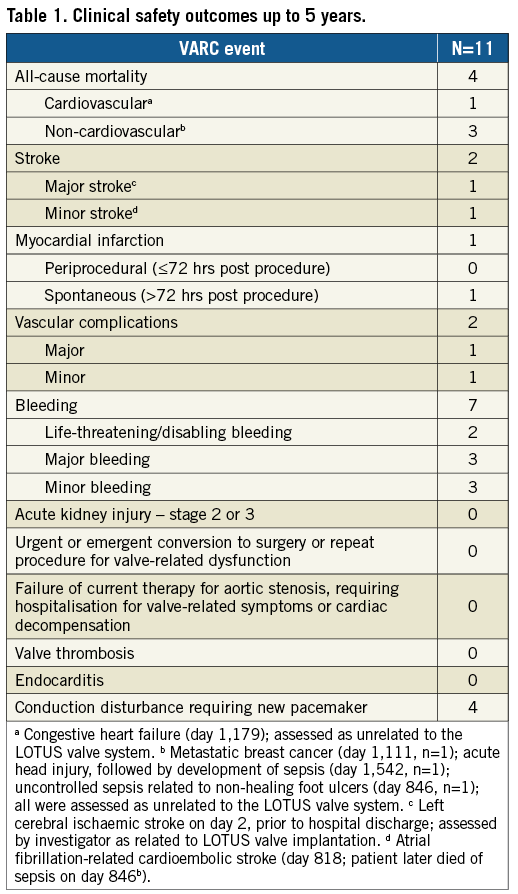
Patients exhibited sustained improvement in haemodynamic performance (Figure 1). Paravalvular aortic regurgitation, as evaluated by an independent echocardiographic core laboratory, was absent or trace in all evaluable patients (Figure 2). At baseline, patients presented in either New York Heart Association (NYHA) functional Class II (54.5%) or Class III (45.5%). Five years after TAVR, 4/6 patients (66.7%) were NYHA Class I, one patient was Class II, and one patient was Class III (in the final year of follow-up, this patient experienced a non-ST-segment elevation myocardial infarction and was diagnosed with decompensated left ventricle failure; these events were not considered to be related to the LOTUS valve system).
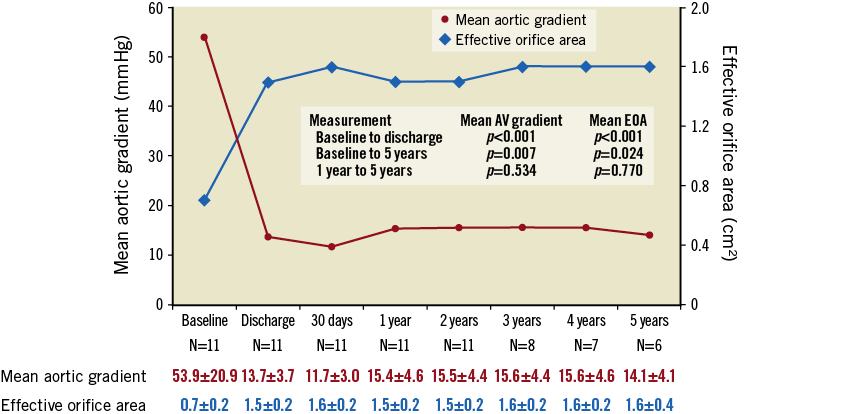
Figure 1. Echocardiographic outcomes up to five years. Patients exhibited sustained improvement in haemodynamic performance. Core laboratory-adjudicated data; p-values from repeated measures and random effects ANOVA model.
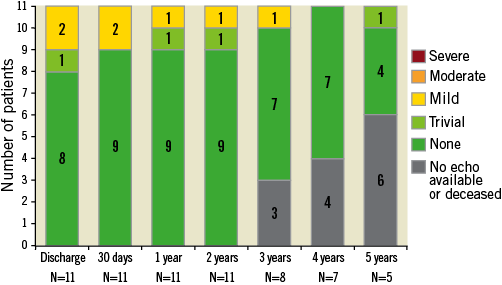
Figure 2. Aortic valve regurgitation. There were no cases of moderate or severe paravalvular regurgitation following LOTUS valve implantation. Core laboratory-adjudicated data.
Discussion
Although TAVR has grown in popularity as a treatment option for aortic valve stenosis, it remains a relatively young field, and long-term data are limited. The REPRISE I study represents the longest follow-up to date for patients treated with the fully repositionable and retrievable LOTUS valve.
Study limitations
A key limitation of this feasibility study is that it was a single-arm study performed in a small number of patients; however, a number of ongoing studies will provide longer-term data on use of the LOTUS valve in patients with aortic valve stenosis. Final five-year data from the REPRISE II study and REPRISE II Extension cohort (n=250 patients) are anticipated in 2019. Additionally, the RESPOND post-market registry, which represents the largest patient population treated with the LOTUS valve in routine clinical practice (n=996 patients), will follow patients annually for five years post implantation.
Conclusion
Final five-year results from the REPRISE I study demonstrate favourable valve function with minimal paravalvular regurgitation following TAVR with the LOTUS valve.
| Impact on daily practice Data from the REPRISE I study support the sustained safety and performance of the LOTUS valve for the treatment of patients with severe aortic stenosis. |
Acknowledgements
The authors thank Victor Davila-Román, MD (CVR Consulting), for echocardiographic analysis; Hong Wang, MS, and MaryEllen Carlile Klusacek, PhD (both of Boston Scientific Corporation), who provided statistical analysis and assistance in manuscript preparation, respectively.
Funding
The REPRISE I study was sponsored and funded by Boston Scientific Corporation. ClinicalTrials.gov Identifier: NCT01383720.
Conflict of interest statement
R. Gooley reports personal fees from Boston Scientific and Medtronic. S. Worthley reports grants from St. Jude Medical, and grants and personal fees from Medtronic. A. Newcomb reports personal fees from Edwards Lifesciences. D. Allocco and I. Meredith report employment at and equity ownership in Boston Scientific. The other authors have no conflicts of interest to declare.

