Abstract
Background: In the last decade, transcatheter aortic valve implantation (TAVI) has developed rapidly in China. As TAVI progresses towards low surgical risk patients, the total number of TAVI procedures will grow exponentially. There is a need to develop a domestic TAVI device designed for Chinese patients.
Aims: The aim of this study was to evaluate the safety and efficacy of a self-expanding valve (TaurusOne transcatheter aortic valve system) in the treatment of patients with symptomatic severe aortic stenosis in China.
Methods: A prospective, multicentre, single-arm study was designed to enrol 120 patients with symptomatic severe aortic stenosis undergoing TAVI using the TaurusOne valve. The primary endpoint was all-cause mortality at one year.
Results: From September 2017 to April 2019, 120 patients were enrolled (35% bicuspid aortic valve, mean Society of Thoracic Surgeons [STS] score 9.95%). One-year mortality in 120 patients (follow-up rate, 100%), was 6.7% (upper 95% confidence interval: 12.9%), which was significantly lower than the performance goal of 30% (p<0.0001). All stroke, myocardial infarction, paravalvular leak ≥moderate, and new pacemaker implantation occurred in 4.4%, 1.8%, 0.8%, and 22.1% of patients, respectively, at one year. The haemodynamic results and quality of life scores also improved significantly. Patients with a bicuspid valve had similar outcomes to those with a tricuspid aortic valve.
Conclusions: The one-year clinical results confirm the safety and efficacy of the TaurusOne transcatheter aortic valve system in the treatment of patients with symptomatic severe tricuspid and bicuspid aortic stenosis.
Introduction
Based on data from numerous randomised controlled trials, transcatheter aortic valve implantation (TAVI) has already been shown to be a safe and effective therapy for patients with symptomatic severe aortic valve stenosis (AS)12345. In the last decade, TAVI has developed rapidly in China with nearly 5,000 patients treated using first-generation self-expanding devices. As TAVI progresses towards low surgical risk patients, the total number of TAVI procedures will grow exponentially67. Given the relatively young age of patients undergoing TAVI in China, bicuspid aortic valve (BAV) disease is encountered with greater frequency and is associated with greater procedural and technical challenges89. There is a need, therefore, to develop a domestic TAVI device designed specifically for Chinese patients.
The TaurusOne transcatheter aortic valve replacement system (Peijia Medical) was developed while keeping in mind the anatomical challenges associated with Chinese patients with severe aortic valve calcification and bicuspid pathology. The objectives of this prospective, multicentre, single-arm study were to evaluate the safety and efficacy of the TaurusOne transcatheter aortic valve system in patients with severe, calcified aortic stenosis.
Methods
Study design
From September 2017 to April 2019, a prospective, multicentre, single-arm study was conducted at 6 centres in China (Fuwai Hospital, The 2nd Affiliated Hospital of Zhejiang University, West China Hospital of Sichuan University, General Hospital of Northern Theater Command, The 2nd Xiangya Hospital of Central South University and The 2nd Affiliated Hospital of Harbin University). Definitions for the study clinical endpoints were based upon the Valve Academic Research Consortium-2 (VARC-2) definitions10. The primary endpoint was all-cause mortality at 12 months. The secondary efficacy endpoints included haemodynamics, New York Heart Association (NYHA) Class and the EuroQol five dimensions questionnaire (quality of life [Qol]) at one year. The safety endpoints included the need for a second valve, conversion to surgery, valve malposition, all stroke, myocardial infarction, new pacemaker implantation, coronary obstruction and paravalvular leak (PVL) ≥moderate at one year. Clinical assessments, chest X-rays, electrocardiography and transthoracic echocardiography were performed at baseline and at follow-up periods of 6 and 12 months. Multislice computed tomography (MSCT) was performed at baseline. Patient inclusion criteria included: (1) providing signed informed consent, (2) age ≥70 years, (3) severe calcified native aortic stenosis by echocardiography (peak velocity ≥4.0 m/s, pressure gradient ≥40 mmHg, orifice area <0.8 cm2, or effective orifice area index <0.5 cm2/m2), (4) symptoms of NYHA Class ≥Ⅱ attributed to AS, (5) prohibitive risk for surgical aortic valve replacement assessed by the Heart Team (Society of Thoracic Surgeons [STS] score ≥8), (6) life expectancy ≥12 months, (7) annulus diameter (MSCT) ≥18 mm and ≤29 mm, ascending aortic diameter <50 mm. The main exclusion criteria included: patients with an unsuitable aortic root or access anatomy, patients with former aortic valve replacement, left ventricular ejection fraction (LVEF) <20%, severe mitral or tricuspid valve regurgitation, left ventricular outflow tract (LVOT) obstruction and evidence of an acute myocardial infarction ≤30 days before the intended treatment. Patients with surgical bioprosthesis failure were also excluded from this study as the surgical aortic valve replacement procedure may significantly affect the morphological characteristics between the bioprosthesis and native valves, which may subsequently influence the TAVI procedure. The final decision to proceed with TAVI was made by a dedicated Heart Team composed of experienced clinical and interventional cardiologists, imaging specialists, cardiovascular surgeons, and anaesthesiologists. The protocol and consent forms were approved by site-specific institutional review boards.
Device description
The TaurusOne transcatheter aortic valve comprises a self-expanding Ni-Ti stent and a trileaflet bovine pericardial valve (Supplementary Figure 1). The stent has a cone-shaped inflow end with high radial force for patients with severe calcification and BAV. The bovine pericardial material is specially processed for anti-calcification treatment. The short frame and low-position valve are designed to reduce coronary obstruction risk, while the inflow end with a polyethylene terephthalate (PET) skirt is designed to reduce paravalvular aortic regurgitation. Low density and large cells at the ascending aorta part are designed to prevent coronary obstruction and provide easy re-access to the coronary artery after TAVI. The valve prosthesis is manufactured in four different sizes (23, 26, 29, and 31 mm) for implantation in native aortic annulus diameters ranging from 18 to 29 mm. The non-retrievable system can be released by an ergonomic handle, which is designed for delicate fast/slow deployment. A capsule with an outer diameter of 18 Fr is utilised for low-profile delivery (Supplementary Figure 1).
Procedure
Statistical analysis
For objective performance criteria (OPC) study, a prespecified objective value for the primary endpoint of all-cause mortality at 12 months had been set at 30%, and an expected target value of 18% was chosen based on previous studies of first-generation TAVI devices23. Considering 10% of patients would probably be lost to follow-up, a total of 120 patients was needed to achieve 80% power in proving non-inferiority to OPC. (Note: this clinical trial was submitted to the China National Medical Products Administration [NMPA] as product registration research, and the 80% power met the usual requirement of clinical research in China). Continuous variables and categorical variables are presented as mean±SD (range) and percentages. Time-to-event analyses were performed using the Kaplan-Meier method. Comparisons between the two groups (tricuspid and bicuspid) were performed using the chi-square test or Fisher’s exact test for categorical variables, and the independent t-test for continuous covariates. All p-values were based on two-sided tests and were considered to be statistically significant at p<0.05. Statistical analyses were performed using SPSS, Version 20.0 (IBM Corp.).
Results
Baseline characteristics
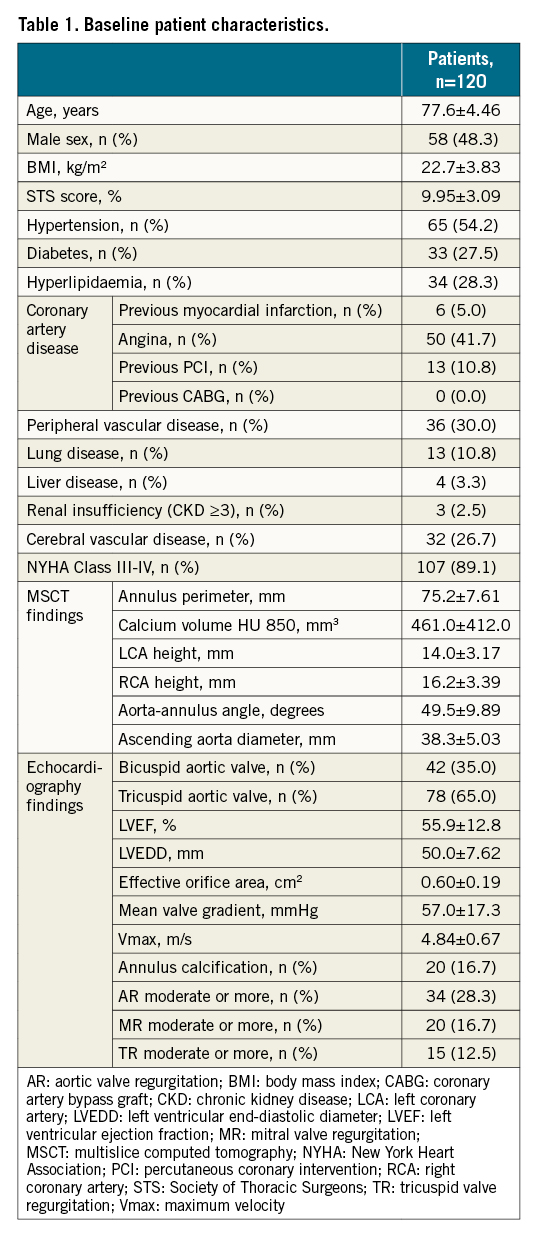
A total of 120 patients from 6 centres (mean age 77.6±4.46 years) were enrolled and underwent TAVI with the TaurusOne transcatheter system between September 2017 and April 2019 (Supplementary Figure 2). Baseline characteristics are listed in Table 1. Mean STS score was 9.95±3.09%. The median and IQR values of the STS score were 9.10 (8.20-10.29%). One out of 120 patients had an STS score lower than 8%. Due to lung disease, this case was judged to be high-risk for a surgical procedure by the Heart Team and was included in this study. According to cardiac imaging, 42 (35%) patients had BAV morphology, and 105 patients (87.5%) were classified as having moderate-severe calcium through echocardiography. The mean perimeter of the aortic annulus was 75.3±7.61 mm.
Perioperative and 30-day follow-up results
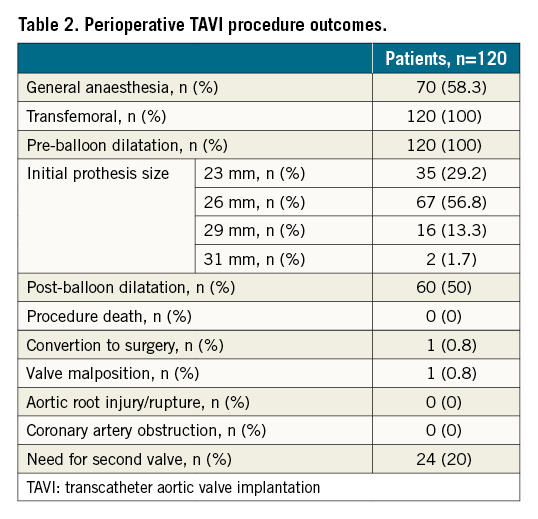
The perioperative TAVI results are shown in Table 2. The majority (58.3%) of patients underwent general anaesthesia. All patients underwent predilation. More than half of the patients (57%) were implanted with a 26 mm valve, 29% with a 23 mm, 13% with a 29 mm, and 2% with a 31 mm valve. A total of 117 (98%) cases had been accessed with the bioprosthesis placed in a suitable anatomical position and met the expected haemodynamic index. One valve was implanted in 80% of patients, while 20% of patients required 2 valves (valve-in-valve) because of an unsatisfactory position of the initial valve. One case had valve malposition and 1 case converted to open heart surgery during TAVI; 3 cases had moderate PVL after TAVI. There were no procedural deaths and no cases of coronary artery occlusion. At 30-day follow-up, 1 patient had died of heart failure, 1 patient had had a major stroke, and 3 patients had moderate PVL. There were no other adverse events at 30 days.
Twelve-month follow-up outcomes
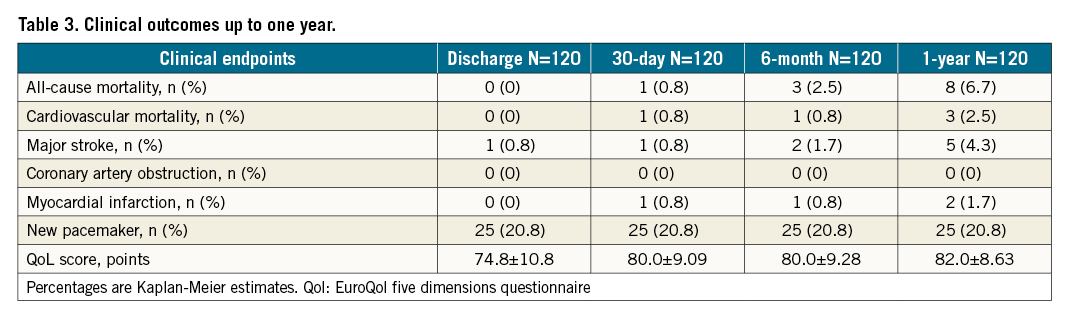
No patients were lost to follow-up at 12 months (Table 3). The primary endpoint of all-cause mortality at one year was 6.7% (upper 95% confidence interval [CI]: 12.9%) which was significantly lower than the pre-set performance goal of 30% (p<0.0001) (Central illustration). The cardiovascular mortality was 2.5% at one year (Supplementary Figure 3). Symptomatic improvement (NYHA Class ≤II) was documented in 96% of patients (Figure 1) and the QoL score increased to 82.0±8.63 at one year (Table 3). At one-year follow-up, echocardiography recorded a mean transaortic valve gradient of 11.8±5.76 mmHg and a mean effective orifice area of 1.83±0.60 cm2 with no significant change from 30-day or 6-month follow-up. LVEF remained stable at one year (Figure 2, Supplementary Figure 4). At 30-day follow-up, moderate PVL was observed in 3 patients (Figure 3). Stroke occurred in 4.4% of patients and myocardial infarction occurred in 1.8% at one-year follow-up. Due to the high proportion of BAV and severe calcification on the leaflets, the prosthesis implant position was more likely to slide towards the LVOT for the first-generation device. A total of 22.1% of patients had required a new pacemaker implantation by one-year follow-up.
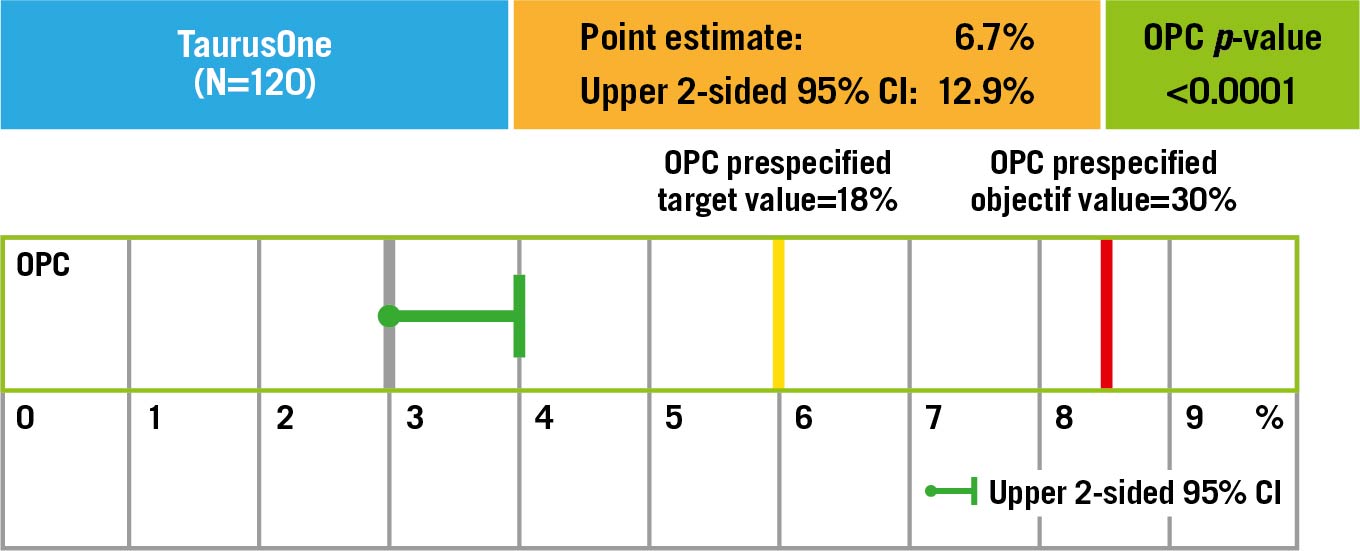
Central illustration. The pre-set objective performance goal. The primary endpoint, all-cause mortality at one year in 120 patients (follow-up rate, 100%), was 6.7% (upper 95% confidence interval [CI]: 12.9%), which was significantly lower than the performance goal of 30% (p<0.0001). OPC: objective performance criteria
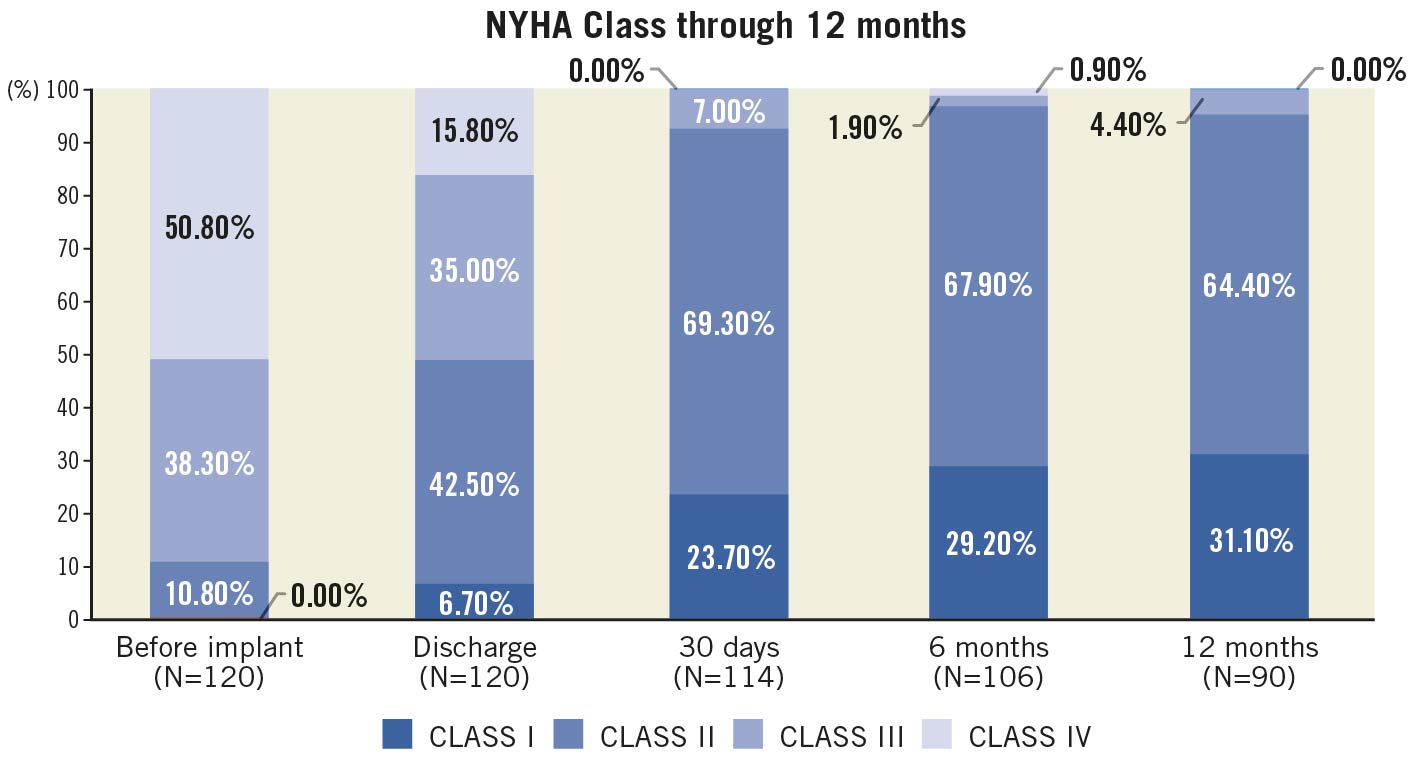
Figure 1. NYHA outcomes up to 12 months. Haemodynamic parameters, NYHA function and PVL at baseline and follow-up.
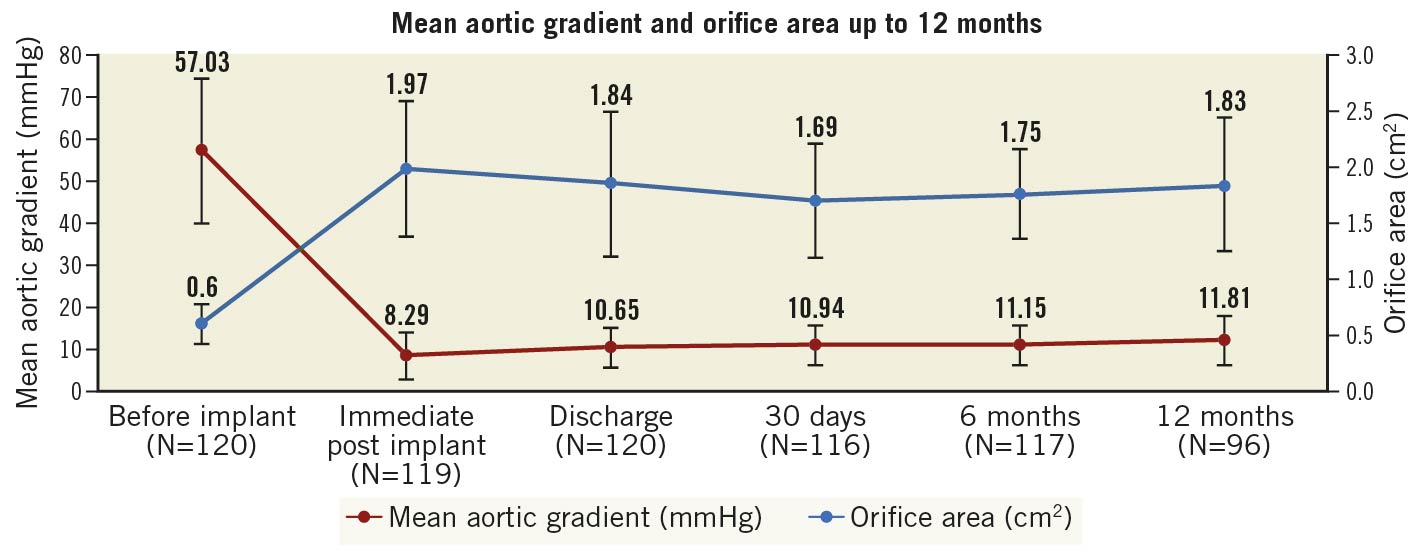
Figure 2. Mean aortic gradient and orifice area up to 12 months. Haemodynamic parameters, NYHA function and PVL at baseline and follow-up.
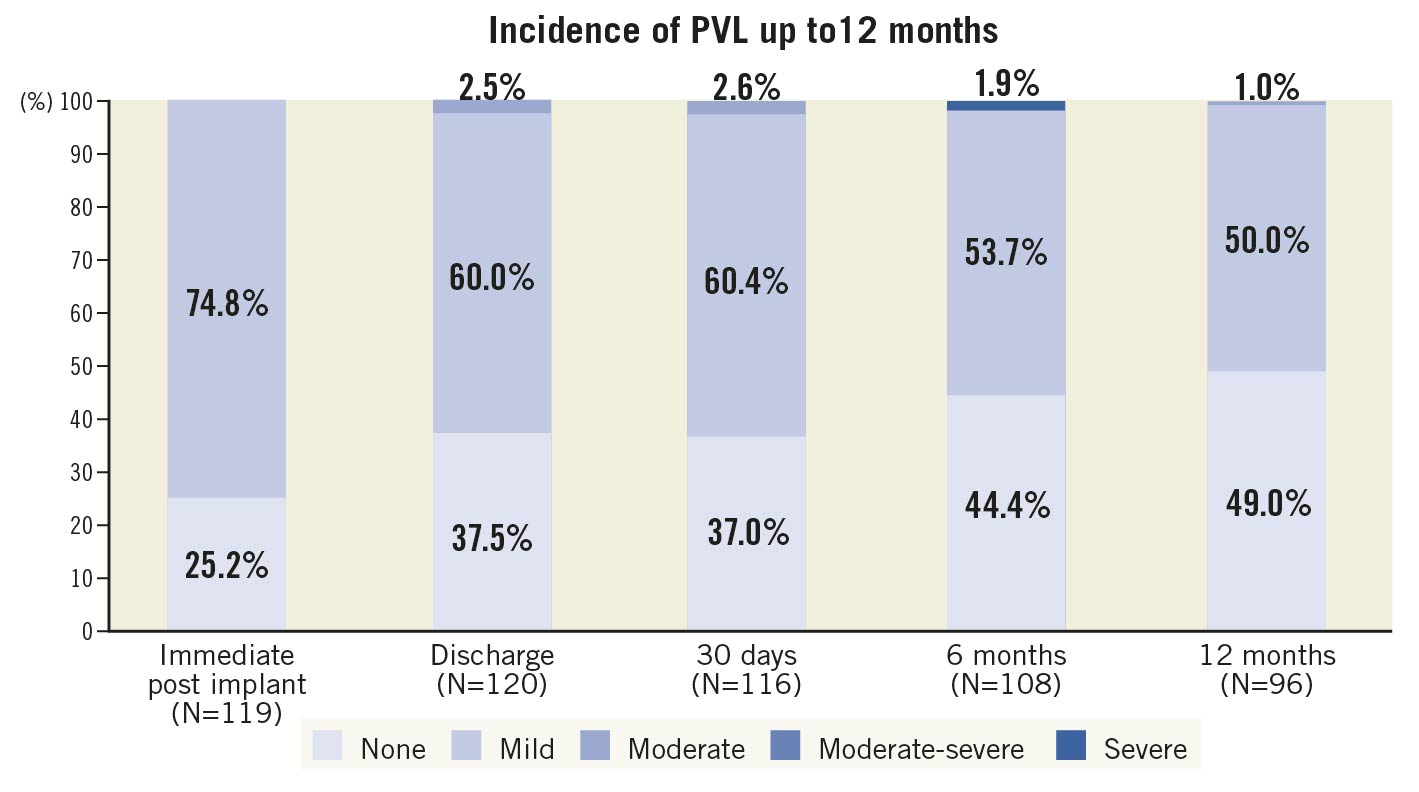
Figure 3. Incidence of PVL up to 12 months. Haemodynamic parameters, NYHA function and PVL at baseline and follow-up.
Performance in bicuspid patients
Based on the imaging evaluation, 42 (35%) patients had BAV morphology while the other patients had tricuspid aortic valve (TAV) morphology. As described in Supplementary Table 1, there were no significant differences between the two groups with respect to baseline characteristics. Patients with BAV and TAV stenosis had similar one-year clinical outcomes, indicating that the TaurusOne transcatheter system showed good performance in BAV patients as well as in TAV patients.
Discussion
This prospective safety and efficacy study included 120 patients with severe AS from 6 centres across China who underwent TAVI with the TaurusOne. There were no procedural deaths and the 1-year all-cause mortality, 1-year cardiovascular mortality, and the 1-year all stroke rates were 6.7%, 2.5%, and 4.4%, respectively. Almost 1 in 3 patients (35%) had BAV; the clinical outcomes at 30 days and 1 year between BAV and TAV were similar.
The mean STS score of patients in our study was 9.95±3.09%, which is the highest among TAVI patients studied in China1112. In light of this being the first-generation TaurusOne transcatheter aortic valve, the results compare favourably with the first-generation SAPIEN (Edwards Lifesciences) and CoreValve (Medtronic) transcatheter aortic valves in high-risk surgical populations. The mean STS score in the PARTNER IA study was 11.3% and was associated with one-year all-cause mortality, cardiovascular mortality, and all stroke rates of 24%, 14%, and 6%, respectively2. The CoreValve High Risk study reported a mean STS score of 7.3% and was associated with a one-year all-cause mortality of 14%3. Similar to Europe and North America, first-generation TAVI devices in China were being implanted by new operators.
The treatment of patients with severe calcification and bicuspid aortic valves requires that transcatheter aortic valves, such as the TaurusOne, have sufficient radial force to maintain effective orifice areas and reduced gradients. The valve area and gradients in the current study were significantly improved and maintained up to one-year follow-up after TAVI. Although 23/26 mm prostheses were used more frequently (86.0%) than the 29 mm in this study, the valve orifice area at one year (1.83 cm2) compares favourably to those studies with the Evolut self-expanding valve (Medtronic; 37.5% of prostheses were 23/26 mm, valve orifice area was 1.88 cm2)3. At the same time, the LVEF, the NYHA classification of cardiac function, and the QoL score also improved significantly during the one-year follow-up in the study population.
Our study showed that the incidence of stroke, vascular complications, and myocardial infarction was low within one year after TAVI, despite the TaurusOne valve being a first-generation self-expanding valve. This was comparable to the Evolut Low Risk study using a retrievable self-expanding valve3. In the Evolut Low Risk study, the incidence of one-year stroke was 4.1%, the vascular complication rate was 3.8%, and the myocardial infarction rate was 1.7%. Despite the early learning curve of TAVI and non-retrievable devices in China, only one patient had moderate PVL during the one-year follow-up period, lending credence to the advantages of high radial force and a PET skirt to prevent PVL.
The rate of implantation of a second valve in the current study was high. Given that the first-generation TaurusOne is non-retrievable, the severe calcification in the supra-annular space probably created downward migration forces during valve deployment1314. It is expected that, with increasing operator experience and the development of a retrievable system, the rates of using a second valve will decrease dramatically1516. Nevertheless, we found that the implantation of a second valve did not affect the haemodynamic results or mortality at one-year follow-up.
During nearly 10 years of investigation, the morphological characteristics of Chinese TAVI patients have aroused widespread attention. The proportion of BAV in Chinese TAVI patients has reached 40%, which is significantly higher than that of western populations due to the overall younger age of Chinese TAVI patients. In this study, the TaurusOne valve showed similar results among patients with BAV and TAV stenosis during one year of follow-up after TAVI, especially in terms of the effect of valve orifice area, gradients and PVL. This first-generation non-retrievable valve has proved that it can also achieve satisfactory results in BAV patients17.
Limitations
This was not a randomised controlled study and the relatively small sample size prevented a more robust analysis of differences in outcome. The study did not incorporate an echocardiographic and CT core lab, but all the centres had an experienced Heart Team focusing on data collection and peri-operation analysis. In addition, the methods of measurement were taught and standardised to ensure the accurate and standardised evaluation of each patient. BAV classification and degree of calcification were determined mainly by echocardiography, and the clinical data did not include statistical analysis of the BAV classification and calcification score by CT. Moreover, the follow-up time was limited to 12 months. Longer follow-up is needed to observe the efficacy and durability of the valve.
Conclusion
This study met its performance goal and demonstrated the safety and efficacy of the TaurusOne transcatheter valve system in treating high surgical risk calcified AS patients. The population in this study had a high prevalence of BAV and severe calcification. The results showed excellent clinical outcomes and improvements in haemodynamic and functional status that were sustained at 12-month follow-up both in BAV and in TAV.
Impact on daily practice
The one-year results of the China TaurusOne valve TAVI study were acceptable, indicating that the TaurusOne transcatheter aortic valve system is a promising device for treating patients with symptomatic severe calcified aortic valve stenosis.
Funding
This study was supported by Peijia Medical (Suzhou) Co., Ltd, China.
(CFDA Medical Device Clinical Trials No. 2017L0003).
Acknowledgements
Conflict of interest statement
N. Piazza declares being a consultant/proctor for HighLife, Medtronic and Peijia. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

