
Abstract
Aims: Transcatheter aortic valve implantation (TAVI) has gained rapid acceptance for patients with severe aortic stenosis (AS) at high surgical risk for conventional valve replacement. Although TAVI is now a relatively mature technique, limited data about long-term valvular function are available. Our aim was to report the five-year echocardiographic data evaluating valve performance from three early European feasibility studies designed to assess the safety and effectiveness of the first-generation balloon-expandable transcatheter heart valve (SAPIEN THV).
Methods and results: A total of 410 patients were enrolled in the following single-arm, non-randomised, prospective multicentre clinical studies: REVIVE II, TRAVERCE and PARTNER EU. Five-year follow-up was completed in 114 surviving patients. Mean patient age was 82.3±5.6 years; 63.4% were female. The mean logistic EuroSCORE was 28.4±13.3%. NYHA Class III/IV was reported in 92.5%. At five years, the mean effective orifice area (EOA) was 1.6±0.6 cm² (n=34) and the mean gradient was 11.7±5.4 mmHg (n=39). In paired patient data, the difference between discharge and five-year EOA was 0.1±0.7 cm² (p=0.3956) and mean gradient was 2.2±5.7 mmHg (p=0.0900). At discharge and five years, respectively, aortic regurgitation (AR) was evaluated as none/trace in 66.6% (n=162/243) and 55.3% (n=19/38), mild in 28.4% (n=69/243) and 39.5% (n=15/38), and moderate in 4.9% (n=12/243) and 5.3% (n=2/38). No severe AR was reported at follow-up. Valve thrombosis was observed in three patients and occurred within one year. No valve-related explants and no case of structural valve deterioration have been reported.
Conclusions: Long-term echocardiographic outcomes in high-risk patients with severe AS suggest stable haemodynamic function of first-generation balloon-expandable SAPIEN THVs at five years, with no worsening of AR severity over time.
Abbreviations
AR: aortic regurgitation
AS: aortic stenosis
EOA: effective orifice area
NYHA: New York Heart Association
RVP: rapid ventricular pacing
TAVI: transcatheter aortic valve implantation
TEE: transoesophageal echocardiography
THV: transcatheter heart valve
VARC: Valve Academic Research Consortium
Introduction
Transcatheter aortic valve implantation (TAVI) has gained rapid acceptance for patients with severe aortic stenosis (AS) who are at high risk for conventional aortic surgery. Short-term and intermediate-term outcomes have been encouraging1-4. However, data on long-term clinical outcomes, valve durability, valve performance and structural integrity remain limited5,6. Even though the five-year outcomes of the balloon-expandable Edwards SAPIEN transcatheter heart valve (THV) (Edwards Lifesciences, Irvine, CA, USA) from the PARTNER trial7,8 and the five-year outcomes of the self-expanding CoreValve® prosthesis (Medtronic, Minneapolis, MN, USA) in a single Italian centre9 were recently reported, additional data on valve durability remain essential before the indication is further expanded to intermediate and lower-risk patients.
The objective of this pooled analysis was to assess five-year echocardiographic valve outcomes using a first-generation balloon-expandable THV.
Methods
STUDY DESIGN AND ENDPOINTS
This is a pooled analysis from three European trials: REVIVE II, TRAVERCE, and PARTNER EU, which were non-randomised, multicentre, feasibility trials designed to evaluate the safety and effectiveness of first-generation balloon-expandable SAPIEN THVs in high-risk and inoperable patients. A total of 410 intention-to-treat (ITT) patients were enrolled in the trials: REVIVE II (transfemoral approach; n=106), TRAVERCE (transapical approach; n=173), and PARTNER EU (transfemoral and transapical approaches; n=131) (Table 1). Of these enrolled patients, 388 were implanted with a SAPIEN THV (23 or 26 mm) (valve implant [VI] population): REVIVE II, n=95; TRAVERCE, n=173; PARTNER EU, n=120.
Five-year clinical and echocardiographic follow-up was completed in 114 surviving patients (REVIVE II, n=25; TRAVERCE, n=49; and PARTNER EU, n=40). The protocol was approved by the ethics committee at each site. Each patient signed an informed consent for data collection and analysis. Procedural, mortality and clinical endpoints were not defined according to the Valve Academic Research Consortium (VARC) criteria, as they were not yet established at the time of the study. The clinical endpoints of valve efficacy, safety, and success are the focus of this analysis. Post-implant aortic regurgitation (AR) represented an important clinical endpoint. The severity of regurgitation was qualitatively assessed and graded according to established guidelines by a central core laboratory. Regurgitation was categorised as paravalvular, transvalvular, or mixed and was classified as none (0), trace (I), mild (II), moderate (III), or severe (IV). The echocardiography data as mentioned were analysed by a dedicated core laboratory.
Valve-related adverse events were collected throughout the five-year observation, and echocardiograms were conducted pre and post procedure, at discharge and at years one to five.
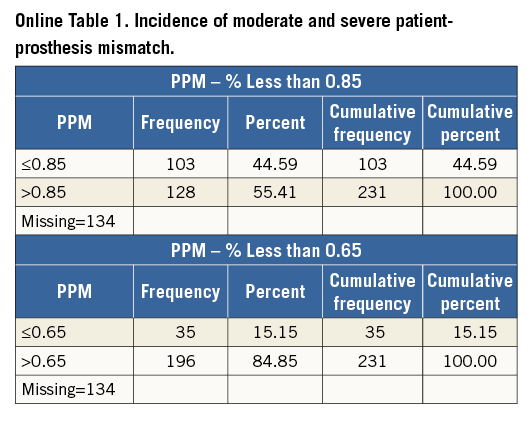
We also assessed for the presence of patient-prosthesis mismatch (PPM) and its impact on mortality. The first available post-implant (discharge, 30 days, or six months) echocardiogram showing effective orifice area (EOA) indexed for BSA was used to identify and quantify PPM. The severity of PPM was graded from the echocardiograms using the indexed EOA, with absence defined as >0.85 cm2/m2, moderate defined as ≥0.65 and ≤0.85 cm2/m2, and severe defined as <0.65 cm2/m2. Results were pooled across the three studies.
PROCEDURES
The design of the first-generation SAPIEN THV balloon-expandable system and the TAVI procedure have been described previously2. Transfemoral procedures were performed under conscious sedation or general anaesthesia with transoesophageal echocardiography (TEE) at the discretion of the implanting team. After retrograde predilation of the native valve, the THV was advanced by the RetroFlex catheter (Edwards Lifesciences), positioned within the native aortic valve, and then delivered by balloon inflation under rapid ventricular pacing (RVP). For the TA procedure, a left anterolateral minithoracotomy and pericardiotomy were performed, and a double pledgeted purse-string suture or U stitches were placed at the left ventricular apex. After puncture of the apex, antegrade crossing, and predilatation, the THV was deployed under RVP.
STATISTICAL ANALYSIS
Echocardiographic analyses were carried out for the intention to treat. Categorical variables were compared using Fisher’s exact tests. Continuous variables are presented as means±standard deviation. Paired sample t-tests were used for comparisons of continuous variables between periods. Time-to-event outcomes were assessed using Kaplan-Meier estimates based on all available data and compared with the log-rank test. Survival analysis has been carried out with the Kaplan-Meier method, reporting the incidence of events at each year. A landmark analysis at one year was also performed, and the incidence of the outcomes was assessed with the Kaplan-Meier method from the chosen landmark point. All statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
BASELINE CHARACTERISTICS
Four hundred and ten patients were analysed in the study. The baseline characteristics are shown in Table 2. The mean age of the study population was 82.3±5.6 years with a mean predicted 30-day mortality by logistic EuroSCORE of 28.4±13.3% and a reported New York Heart Association (NYHA) functional Class III or IV of 92.5% at the time of procedure. STS score was available only for the PARTNER EU trial, with a mean predicted 30-day mortality of 11.6±6.5 (IQR: 2.0–41.0).
Clinical follow-up was available in all patients. A total of 114 patients survived during a median follow-up of 60 months. All-cause mortality rates at one, two, three, four and five years were 34.7%, 42.1%, 51.6%, 60.8%, and 68.1%, respectively (Figure 1), and cardiovascular mortality rates were 23.1%, 27.5%, 31%, 36.9% and 39.4%, respectively (Figure 1).
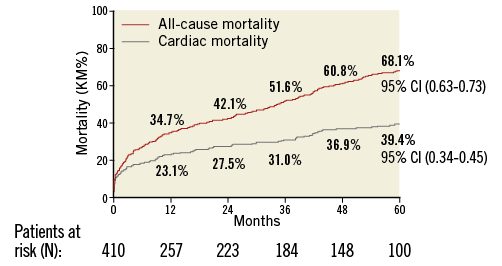
Figure 1. All-cause and cardiac mortality.
Freedom from mortality by trial at five years was 33.1% (PARTNER EU), 32.9% (TRAVERCE) and 28.3% (REVIVE II) (Figure 2). When patients with one-year mortality were excluded, five-year all-cause and cardiovascular mortality were 51.1% and 21.2%, respectively, as shown by the landmark analysis (Figure 3). Causes of cardiovascular and non-cardiovascular death are reported in Table 3 and Figure 4.
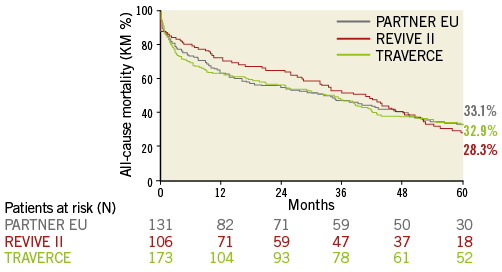
Figure 2. Freedom from mortality by trial.
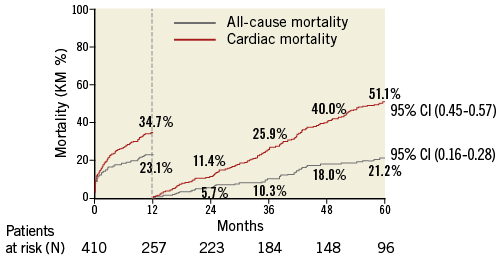
Figure 3. Landmark analysis of all-cause and cardiac mortality.
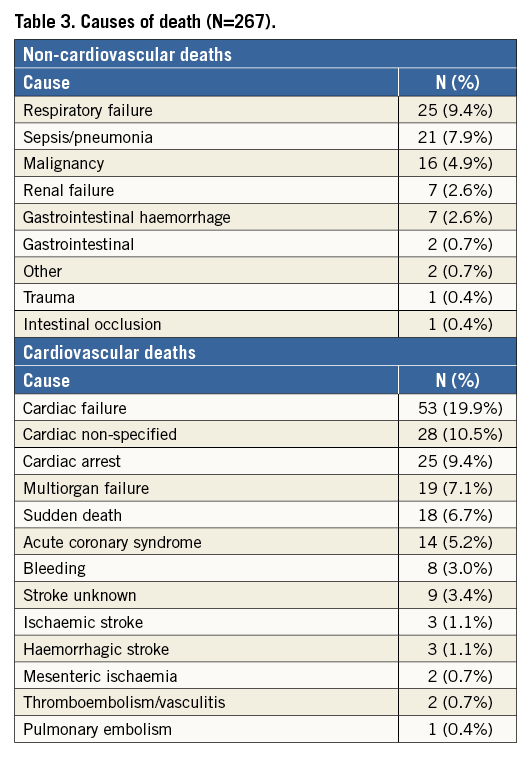
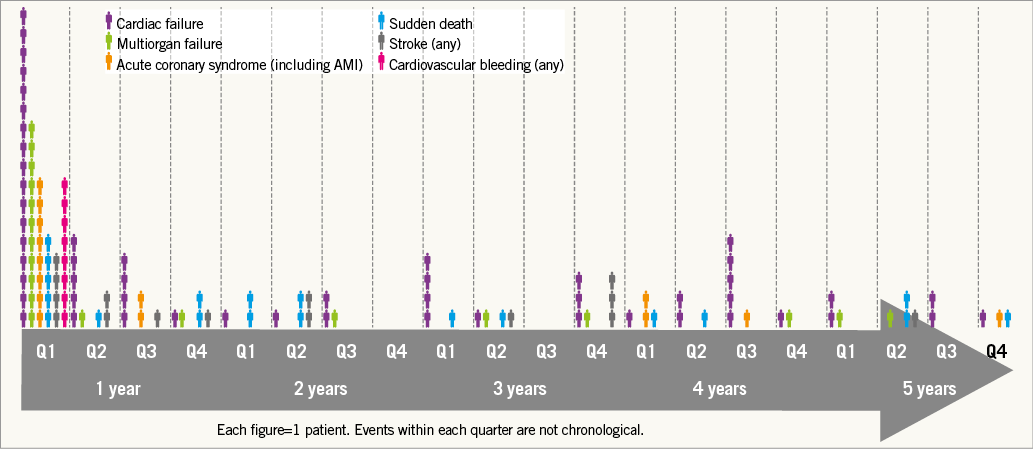
Figure 4. Timeline and causes of cardiac death.
PATIENT PROSTHESIS
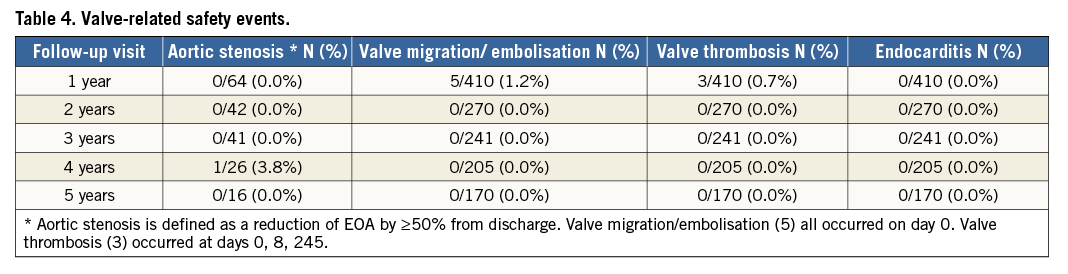
At five years, the mean EOA was 1.6±0.6 cm² (n=34), mean gradient was 11.7±5.4 mmHg (n=39), and peak gradient was 21.2±9.8 mmHg (n=40) (Online Figure 1). In paired patient data, the difference between discharge and five-year mean EOA was 0.1±0.7 cm² (p=0.39) and the difference in mean gradient was 2.2±5.7 mmHg (p=0.09) (Figure 5). Left ventricular ejection fraction (LVEF) at five years was 55.7±16.1% (n=26) (Online Figure 2). At discharge and five years, respectively, aortic regurgitation (AR) was evaluated as none/trace in 66.6% (n=162/243) and 55.3% (n=21/38), mild in 28.4% (n=69/243) and 39.5% (n=15/38), and moderate in 4.9% (n=12/243) and 5.3% (n=2/38) (Figure 6). No severe AR was reported during follow-up. Regarding valve-related safety events, valve thrombosis was observed in three patients (one during index procedure) and occurred within one year, with no cases occurring afterwards. There were five cases of valve embolisation during the procedure but none beyond the index procedure day. No valve-related explants, no prosthesis endocarditis and no case of structural valve deterioration have been reported in our patient population during the five-year follow-up (Table 4). There was only one case noted of valve thickening with EOA of 1 cm2 and a low gradient.
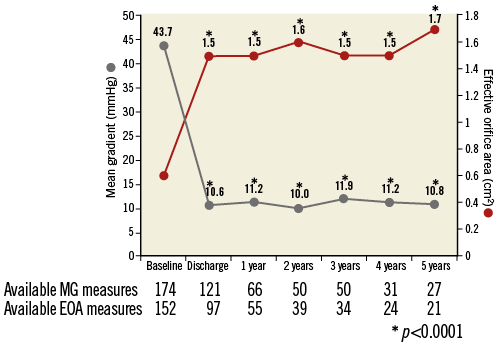
Figure 5. Prosthetic performance: time trends in transaortic gradient and effective orifice area paired at each time point. 1) At discharge, EOA 1.5±0.35, CI: 1.45-1.59, p<0.0001, at one year EOA 1.5±0.33, CI: 1.45-1.63, p<0.0001, at two years EOA 1.6±0.32, CI: 1.45-1.66, p<0.0001, at three years EOA 1.5±0.34, CI: 1.40-1.64, p<0.0001, at four years EOA 1.5±0.31, CI: 1.39-1.65, p<0.0001, at five years EOA 1.7±0.48, CI: 1.49-1.92, p<0.0001. 2) At discharge, mean gradient 10.6±7.80, CI: 9.19-11.99, p<0.0001, at one year mean gradient 11.2±7.62, CI: 9.32-13.07, p<0.0001, at two years mean gradient 10.0±3.61, CI: 8.99-11.04, p<0.0001, at three years mean gradient 11.9±7, CI: 10.43-13.27, p<0.0001, at four years mean gradient 11.2±6.50, CI: 8.88-12.78, p<0.0001, at five years mean gradient 10.8±4.93, CI: 8.82-12.68, p<0.0001.
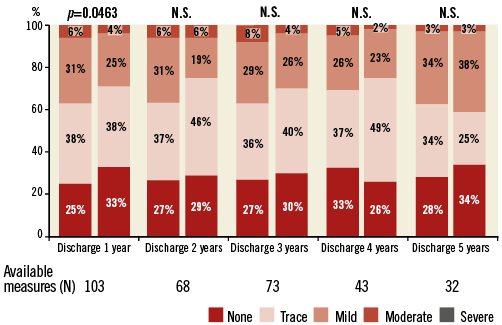
Figure 6. Aortic regurgitation paired at each time interval.
The incidence of moderate and severe PPM was 44.59% (103/231) and 15.15% (35/231), respectively (Online Table 1). Severe PPM was not associated with five-year mortality in our pooled analysis.
Discussion
Data from the PARTNER Trial established that TAVI with balloon-expandable THV is superior to medical therapy in inoperable patients and non-inferior to surgery in patients with AS who are at high risk for conventional aortic valve replacement1,4,10. TAVI with the self-expanding prosthesis was shown to be superior to surgery in high-risk patients11. The most recent 2014 ACC/AHA guidelines have established the role of TAVI as an important alternative in the treatment of patients with severe AS12. Although positive short-term and midterm results with TAVI have been reported1-4, few long-term data on durability are available5-8.
The present pooled analysis from the single-arm, non-randomised, prospective, multicentre European trials describes the five-year outcomes of a cohort of TAVI patients using the first-generation balloon-expandable SAPIEN THV in Europe. The data from these studies demonstrate similar long-term outcomes compared to the PARTNER high-risk cohort with a five-year mortality of 68.1% versus 67.8%, respectively8. Given the high mortality, only one third of patients remained alive and available for haemodynamic analysis at five years. The patients included in this pooled analysis were either inoperable or very high risk for surgery; therefore it is not surprising to see this high mortality at follow-up. It is interesting to note that, beyond the one-year landmark analysis, the majority of deaths were non-cardiovascular (51.1% vs. 21.2%). Furthermore, despite the limited follow-up, no patients died of valve dysfunction during the five years of follow-up. This information is very encouraging for the future inclusion of patients with a lower risk profile.
As the accessibility and duration of implanted THV increase, long-term valve durability and failure become an important issue. Long-term outcomes following THV become even more relevant if the procedure is extended to patients with intermediate and lower risk in view of longer life expectancy. The durability of transcatheter valves remains a concern that requires comprehensive clinical and echocardiography evaluations over an extended follow-up period13. Predisposing factors to adverse long-term outcomes include leaflet trauma from compression within the delivery catheter after balloon dilation, suboptimal leaflet coaptation or leaflet-frame contact due to asymmetrical or incomplete frame expansion, and suboptimal high or low implantation13-15. The five-year results of the randomised PARTNER 1 trial published by Mack and colleagues reported clinical outcomes, valvular structural integrity, and haemodynamic changes in 348 patients treated with the SAPIEN THV and confirmed a favourable medium- to long-term durability and preserved haemodynamic function with no evidence of prosthetic structural failure8. Toggweiler and colleagues reported outcomes in 29 patients who remained alive (88 patients at the start of the study) at five-year follow-up, and demonstrated similar favourable outcomes after TAVI, with signs of moderate prosthetic valve failure in only 3.4% of patients and no cases of severe prosthetic regurgitation5.
Three common THV failures (prosthetic valve endocarditis, structural valve deterioration, valve thrombosis) and two less common ones (late embolisation and compression of the valve during CPR) have been described in the literature13. In this study, the evaluated valve performance was as favourable in the surviving patients analysed up to five years after implantation, with almost no signs of THV failure. The transaortic mean gradients and EOA remained stable over time and no cases of severe paravalvular regurgitation were reported during the five years of observation. There were no cases of valve embolisation, valve endocarditis or leaflet degeneration beyond discharge day up to five years. There were two cases of valve thrombosis reported beyond the index procedure up to five years. Although it was recommended that all patients receive aspirin and thienopyridines prior to procedure and continue thienopyridines for six months post procedure and aspirin indefinitely, we had no information on the anticoagulant regimen at the time of valve thrombosis. Signs of late prosthetic failure were seen in only one patient showing valve prosthetic stenosis at year four, defined in the study as EOA decline more than 50% from baseline. These findings showed similar sustained clinical and echocardiographic outcomes beyond five years compared to the available data from the SAPIEN and SAPIEN XT valves and the self-expanding CoreValve5,7,9.
Although five years may not be sufficient to expect differences in durability between transcatheter and surgical prostheses, it is reassuring, however, that structural valve deterioration in the TAVI population has been minimal while showing stable mean gradients and no worsening in AR severity, even with few patients remaining at risk at five years. Moreover, additional follow-up beyond five years will be challenging owing to the high mortality rates at five years. It is clear that the lack of many late survivors after TAVI is the consequence of treating a high-risk, elderly surgical population with multiple comorbidities, and not the result of prosthetic valve failure7,16,17. As shown in our landmark analysis, the majority of deaths beyond one year were non-cardiac-related mainly due to baseline clinical comorbidities (logistic EuroSCORE, NYHA functional Class III or IV, or COPD). Therefore, this pooled analysis will not answer the question of longer-term durability of the SAPIEN THV, as follow-up was planned to stop at five years. However, the PARTNER Trial will be following patients up to 10 years, and will allow comparison of THV with the long-term durability data already available for the surgical valves.
Several previous studies have reported that severe PPM adversely affects mortality after SAVR18,19. In the PARTNER Trial, PPM was associated with persistence of LV hypertrophy and increased two-year mortality in high-risk patients with severe AS undergoing SAVR20. However, PPM was not associated with increased mortality in the TAVR cohort20. We had a similar rate of severe PPM (15.5%) compared to the PARTNER Trial (19.7%) as well as no association between PPM and long-term mortality. TAVI seems to be preferable to SAVR in small aortic annuli that are predisposed to PPM as it does not seem to affect long-term mortality.
Limitations
This analysis is not without limitations. The trials were designed as single-arm studies and not as a randomised comparison of TA vs. TF approaches, but rather a complementary approach. The three feasibility trials included a “learning curve” for many European TAVI operators and involved procedure refinements and product iterations to address complications. In addition, VARC criteria were not established at the time of the initiation of the trials. Moreover, the surgical risk was assessed by the logistic EuroSCORE rather than the more recently validated EuroSCORE II and STS PROM scores (only available for PARTNER EU), and it was not possible to collect this information retrospectively. A major weakness of this report is that, of the 114 surviving patients, only about 25% of patients had echocardiograms at five years for the analysis. Finally, analyses of echocardiographic data and rates of late prosthetic failure were performed in surviving patients, with death possibly exerting a competing risk that may have biased the results, as valve failures could have occurred in patients who had died before five years. Although there is no suggestion of gradually deteriorating haemodynamics over the sequential years, the data capture was incomplete. Caution is advised when interpreting these data. It should also be noted that clinical outcomes and valve performance in these trials might not reflect those of subsequent generations of balloon-expandable transcatheter valves (SAPIEN XT, SAPIEN 3 THV).
Conclusion
This analysis, in agreement with the PARTNER US study, shows that, at five years, transcatheter balloon-expandable valves offer predictable performance and valve-related outcomes. TAVI with the first-generation balloon-expandable SAPIEN THV appears to be an adequate therapy for high-risk patients with severe aortic stenosis, but longer-term follow-up remains necessary before extending indications to lower-risk and younger patients.
| Impact on daily practice Long-term echocardiographic outcomes in high-risk patients with severe AS suggest stable haemodynamic function of first-generation balloon-expandable SAPIEN THVs at five years. |
Guest Editor
This paper was guest edited by Ran Kornowski, MD, FACC, FESC; Department of Cardiology, Beilinson Hospital - Rabin Medical Center, Petah Tikva, Israel.
Acknowledgements
We are very grateful to Beverly Diamond and Monica Meyer (Edwards Lifesciences) for their assistance in the preparation of this manuscript.
Funding
Edwards Lifesciences sponsored the three trials, and the protocol was developed by the sponsor and the trial steering committee.
Conflict of interest statement
T. Lefèvre, H. Eltchaninoff, M. Romano and W. Wisser are proctors for Edwards Lifesciences. D. Himbert is a proctor and consultant for Edwards Lifesciences. P. Nataf was a proctor for Edwards Lifesciences. M. Thomas is vice-president for THV medical affairs at Edwards Lifesciences. P. Serruys reports personal fees from Abbott Laboratories, AstraZeneca, Biotronik, GLG Research, Medtronic, Cardialysis, Société Europa Digital & Publishing, Sino Medical Sciences Technology Inc., Stentys France, Svelte Medical Systems and Volcano Europe BVBA. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.
Supplementary data
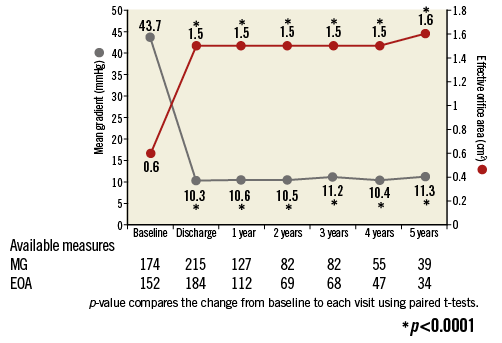
Online Figure 1. Prosthetic performance: time trends in transaortic gradient and effective orifice area. 1) At discharge, EOA 1.5±0.36, CI: 1.45-1.55, p<0.0001, at one year EOA 1.5±0.35, CI: 1.48-1.61, p<0.0001, at two years EOA 1.5±0.34, CI: 1.43-1.59, p<0.0001, at three years EOA 1.5±0.40, CI: 1.43-1.62, p<0.0001, at four years EOA 1.5±0.40, CI: 1.38-1.61, p<0.0001, at five years EOA 1.6±0.60, CI: 1.39-1.81, p<0.0001. 2) At discharge, mean gradient 10.3±6.77, CI: 9.39-11.21, p<0.0001, at one year mean gradient 10.6±6.12, CI: 9.48-11.63, p<0.0001, at two years mean gradient 10.5±7.93, CI: 8.78-12.25, p<0.0001, at three years mean gradient 11.2±4.70, CI: 10.21-12.27, p<0.0001, at four years mean gradient 10.4±5.36, CI: 8.91-11.81, p<0.0001, at five years mean gradient 11.3±5.06, CI: 9.67-13.00, p<0.0001.
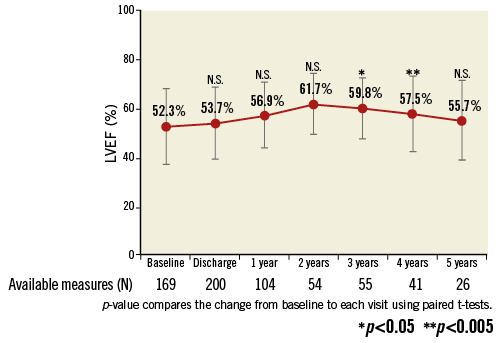
Online Figure 2. Left ventricular ejection fraction.

