Abstract
Background: There are limited head-to-head randomised trials comparing the performance of different transcatheter heart valves (THVs).
Aims: We aimed to evaluate the non-inferiority of the balloon-expandable Myval THV series compared to the balloon-expandable SAPIEN THV series or the self-expanding Evolut THV series.
Methods: The LANDMARK trial randomised 768 patients in a 1:1 ratio, (Myval THV series [n=384] vs contemporary series with 50% SAPIEN THV series [n=192] and 50% Evolut THV series [n=192]). The non-inferiority of Myval over the SAPIEN or Evolut THV series in terms of the 30-day primary composite safety and effectiveness endpoint as per the third Valve Academic Research Consortium (VARC-3) was tested in an intention-to-treat population with a predefined statistical power of 80% (1-sided alpha of 5%) for a non-inferiority margin of 10.44%.
Results: The Myval THV series achieved non-inferiority for the primary composite endpoint over the SAPIEN THV series (24.7% vs 24.1%, risk difference [95% confidence interval {CI}]: 0.6% [not applicable {NA} to 8.0]; p=0.0033) and the Evolut THV series (24.7% vs 30.0%, risk difference [95% CI]: –5.3% [NA to 2.5]; p<0.0001). The incidences of pacemaker implantation were comparable (Myval THV series: 15.0%, SAPIEN THV series: 17.3%, Evolut THV series: 16.8%). At 30 days, the mean pressure gradient and effective orifice area were significantly better with the Myval THV series compared to the SAPIEN THV series (p<0.0001) and better with the Evolut THV series than with the Myval THV series (p<0.0001). At 30 days, the proportion of moderate to severe prosthetic valve regurgitation was numerically higher with the Evolut THV series compared to the Myval THV series (7.4% vs 3.4%; p=0.06), while not significantly different between the Myval THV series and the SAPIEN THV series (3.4% vs 1.6%; p=0.32).
Conclusions: The Myval THV series is non-inferior to the SAPIEN THV series and the Evolut THV series in terms of the primary composite endpoint at 30 days. Clinical trial registration: ClinicalTrials.gov: NCT04275726; EudraCT number 2020-000,137-40.
The LANDMARK trial was the first prospective, randomised controlled trial to show the non-inferiority of a novel platform, the balloon-expandable (BE) Myval (Meril Life Sciences) transcatheter heart valve (THV) series, over contemporary THV series (combined BE SAPIEN [Edwards Lifesciences] and self-expanding [SE] Evolut [Medtronic] THV series)1. In the main analysis, the early clinical outcomes of the Myval THV series were only compared with the combined control group (the Evolut and SAPIEN THV series) with no individual head-to-head comparisons reported1. In the present subanalysis, we report the prespecified, statistically powered comparison between the three individual arms, to provide more granularity to the outcomes. The analytical plan and statistical design enabled us to separately test for the non-inferiority of the Myval THV series against the SAPIEN or Evolut THV series in a separate fashion. Notably, this is also the first randomised comparison of two BE valve (BEV) technologies.
Methods
Study design and participants
This is a powered, predefined substudy of the LANDMARK trial, with an aim to individually assess the non-inferiority of the Myval THV series over the SAPIEN THV series and Evolut THV series for the primary composite safety and effectiveness endpoint at 30-day follow-up.
The LANDMARK trial (ClinicalTrials.gov: NCT04275726) was a prospective, non-inferiority, randomised, open-label trial conducted at 31 centres in 16 countries. It was designed to evaluate the primary safety and effectiveness endpoint reported at 30 days according to the third Valve Academic Research Consortium (VARC-3)2, as well as clinical and haemodynamic outcomes. The trial design, protocol amendment on eligibility criteria, and the main study results have been published previously134. The study was approved by the ethics committees of the respective study sites. Prior to screening, all study participants provided written informed consent. The study was carried out in accordance with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use guidelines.
Adults (≥18 years old) with severe symptomatic native aortic stenosis (AS) were deemed suitable for enrolment if judged by the local Heart Team to be eligible for transcatheter aortic valve implantation (TAVI) utilising all three study devices. A prescreening committee assessed the appropriateness of TAVI with the study THVs using the preprocedural multislice computed tomography (MSCT) provided by an independent core lab (TAVI Core Lab, India). Each site’s Heart Team made the ultimate decision about each subject’s enrolment into the study.
The Myval THV series included Myval and Myval Octacor THVs with sizes 20 mm, 21.5 mm, 23 mm, 24.5 mm, 26 mm, 27.5 mm, and 29 mm in diameter. The SAPIEN THV series consisted of the SAPIEN 3 and SAPIEN 3 Ultra THVs, which were commercially available at the sites, with device sizes 20 mm, 23 mm, 26 mm, and 29 mm in diameter. Lastly, the Evolut THV series included the Evolut R and Evolut PRO THVs or any subsequent advanced commercial version available at the sites with sizes 23 mm, 26 mm, 29 mm, and 34 mm in diameter.
Randomisation and masking
In this trial, 768 severe symptomatic native AS patients were enrolled in a 1:1 ratio to the Myval THV series (n=384) and contemporary THV series (n=384), with subsequent stratification and an equal allocation (1:1) of patients in the contemporary arm between the SAPIEN (n=192) and Evolut (n=192) THV series. A covariate-adaptive randomisation process was used based on the simulation in accordance with the Frane method, considering the power and selection bias concurrently5.
Procedures
The procedural details have been discussed in detail in the earlier publications134. In brief, preprocedural assessment included physical examination, medical history, laboratory investigations, electrocardiography (ECG), echocardiography, and MSCT. The protocol recommended a transfemoral approach. The implantation technique, use of sedation, the need for predilation and post-dilation, and closure of the femoral access (surgical/non-surgical) were left to the operator’s discretion. An aortography was performed at the end of the procedure for offline evaluation of the regurgitation fraction (RF) by videodensitometry67. Whenever a post-dilation was performed, a final aortography was performed and analysed with videodensitometry. The quantitative cutoff of RF% on videodensitometry for moderate-severe regurgitation is ≥17%. Postprocedural ECG, echocardiography, and laboratory investigations were performed. Antithrombotic therapy was recommended according to the European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery (EACTS) guidelines8. At the 30-day follow-up, a clinical assessment, ECG, and echocardiography were performed.
Outcomes
The primary combined safety and effectiveness endpoint at 30 days was a composite of all-cause mortality, all stroke, bleeding (VARC types 3 and 4), acute kidney injury (stages 2, 3, and 4), major vascular complications, moderate or severe prosthetic valve regurgitation (PVR), and conduction system disturbances resulting in a new permanent pacemaker implantation (PPI) as per VARC-32. Secondary endpoints were defined as per VARC-3 and specified in the protocol34; these included the components of the primary endpoint, technical success, device success, and early safety endpoints at 30-day follow-up. The New York Heart Association (NYHA) Functional Class and the 6-minute walk test were used to measure functional improvement, and a 12-item Short Form Survey was used to gauge quality of life.
The primary and secondary endpoints pertaining to technical and device success were evaluated by an independent clinical events committee that was blinded to the randomisation. The Cardiovascular European Research Centre (Paris, France) analysed ECGs, while the CORRIB Core Laboratory (Galway, Ireland) centrally handled echocardiograms and quantitative assessment of aortographic regurgitation.
Statistical analysis
Details of the sample size calculation have been published13. In brief, assuming an event rate of 26.1% and a non-inferiority margin of 10.44%, the sample size of 768 patients was calculated to demonstrate non-inferiority of the Myval THV series to the contemporary THV series (combined SAPIEN and Evolut THV series) with a statistical power of 93% and a 1-sided alpha of 0.05. With this sample size (Myval THV series: n=384, SAPIEN THV series: n=192, Evolut THV series: n=192), the individual comparison of the Myval THV series versus the SAPIEN THV series, and the Myval THV series versus the Evolut THV series, has a statistical power of 80% with a 1-sided alpha of 0.05 to demonstrate non-inferiority of the Myval THV series to the SAPIEN THV series and to the Evolut THV series, respectively. The non-inferiority assessment of the primary endpoint used a 1-sided 95% confidence interval (CI) calculated using the Farrington-Manning test in the intention-to-treat population. For the subsequent superiority analysis and comparison of the itemised primary endpoint, a proportion test was used to compare the difference between the THV types. Continuous variables are summarised using mean±standard deviation (SD) and median (interquartile range [IQR]) according to distribution and were compared using the 2-sample t-test. Categorical variables are presented as frequency (percentage) and were compared using Pearson’s χ² test or Fisher’s exact test, as appropriate. The mean difference and risk ratio of the two arms are presented with 95% CIs. Statistical analysis was performed using R software, version 4.3.3 (R Foundation for Statistical Computing).
Results
Baseline characteristics
Between 6 January 2021 and 5 December 2023, 768 patients with severe symptomatic native AS were enrolled, with 384 participants randomly assigned to the Myval THV series, 192 to the SAPIEN THV series, and 192 to the Evolut THV series (Central illustration). The consort flow diagram is shown in Figure 1. Baseline characteristics, which are tabulated in Table 1, were similar between all three arms. The ages, given as mean±SD, were 80.0±5.7, 81.1±5.4, and 79.7±5.4 years in the Myval, SAPIEN and Evolut THV series arms, respectively. The median (IQR) Society of Thoracic Surgeons score was 2.6% (1.7-4.0) in the Myval THV series arm, 2.6% (1.8-4.0) in the SAPIEN THV series arm and 2.7% (1.5-4.0) in the Evolut THV series arm, indicating on average that all arms included a low-risk population. Of note, all three arms included patients with bicuspid valves (6.0% vs 7.3% vs 7.8%) and small aortic annuli (≤430 mm2; 32.6% vs 33.3% vs 29.2%). Other baseline demographic characteristics were similar in all three arms.
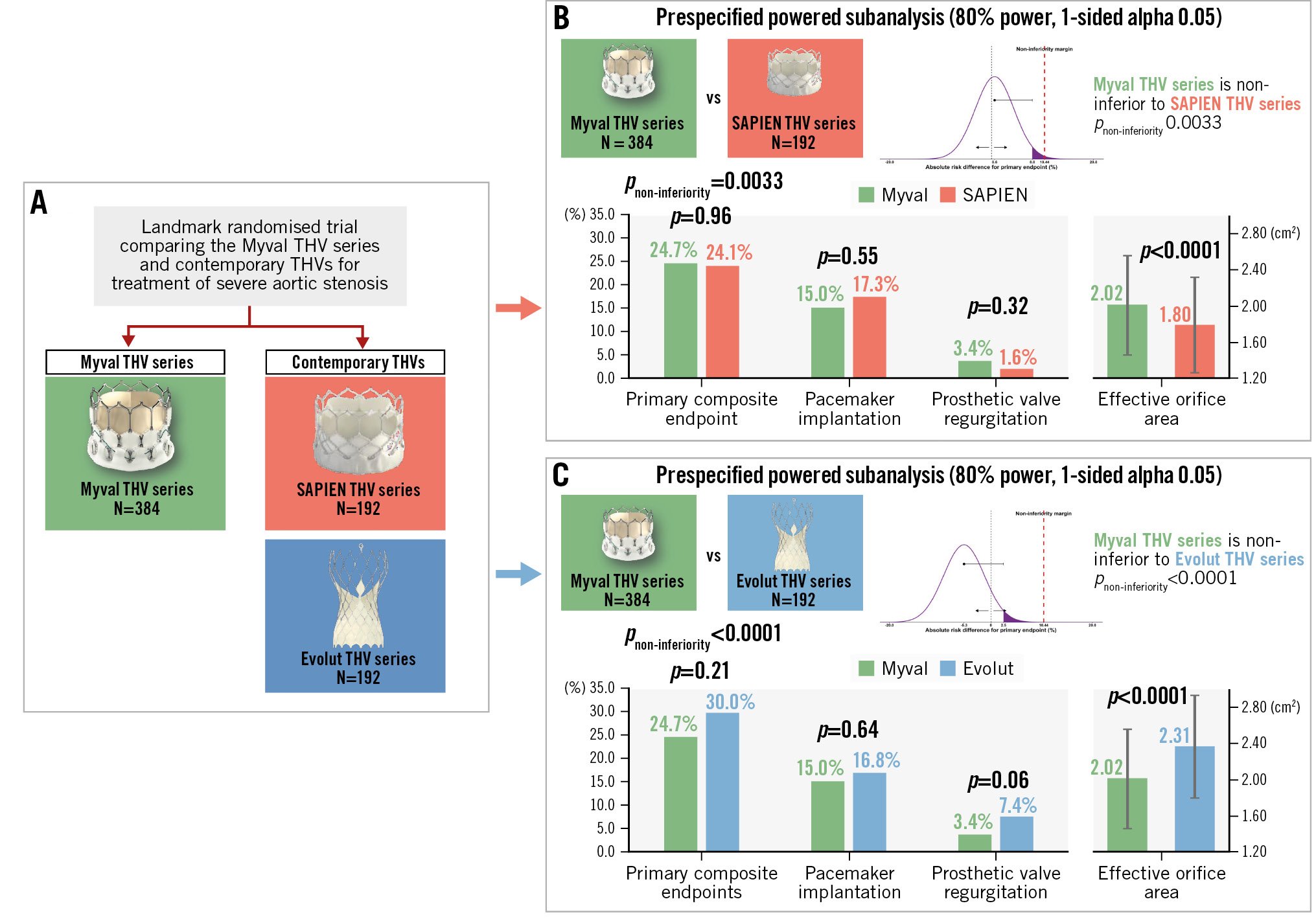
Central illustration. Comparison of clinical and echocardiographic outcomes: Myval vs SAPIEN THV series and Myval vs Evolut THV series. A) The LANDMARK trial, a randomised trial comparing the Myval THV series with contemporary THV series (SAPIEN and Evolut) in patients with severe aortic stenosis. B) Comparison between the Myval and SAPIEN THV series. C) Comparison between the Myval and Evolut THV series. THV: transcatheter heart valve
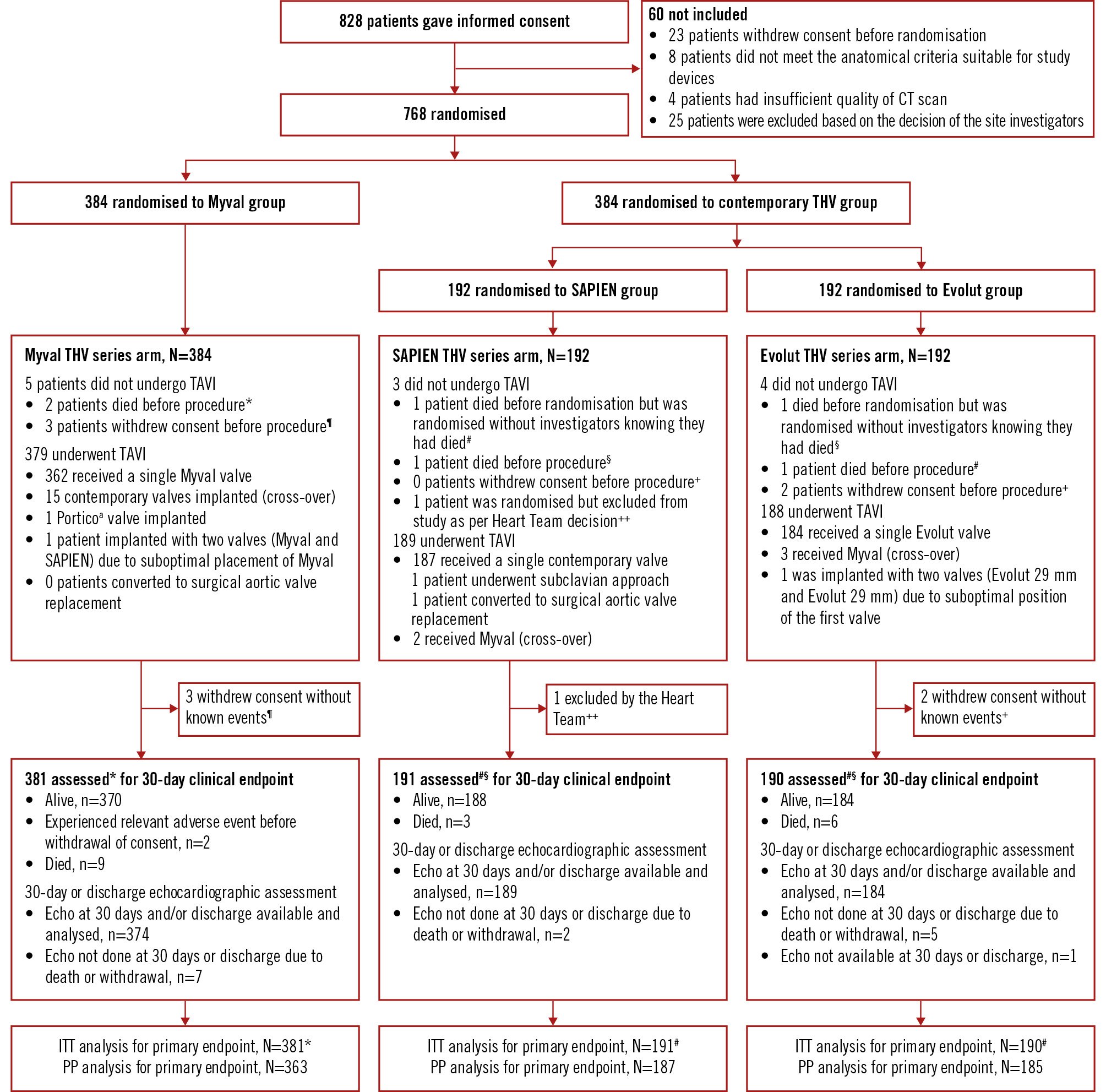
Figure 1. Consort flow diagram of the LANDMARK trial. *Two patients died before the procedure and were included in the population for endpoint analysis. ¶Three patients who withdrew consent before the procedure without any known events at that point in time were excluded from the endpoint analysis. aBy Abbott. #One patient signed informed consent and was randomised; however, the investigators were unaware that the patient had died in the meantime, and therefore the patient was included in the endpoint analysis. §One patient died before the procedure and was included in the population for endpoint analysis. ++One patient was excluded after randomisation by the investigator due to rapid progression of his Alzheimer’s disease. +Two participants who withdrew consent before the procedure without any known events at that point in time were excluded from the endpoint analysis. CT: computed tomography; ITT: intention-to-treat; PP: per-protocol; TAVI: transcatheter aortic valve implantation
Table 1. Baseline characteristics, medical history and cardiac history of all three cohorts in the LANDMARK trial.
| Characteristics | Myvala THV series (n=384) | SAPIENb THV series (n=192) | Evolutc THV series (n=192) |
|---|---|---|---|
| Age, years | 80.0±5.7 | 81.1±5.4 | 79.7±5.4 |
| Sex | |||
| Male | 191 (49.7) | 106 (55.2) | 102 (53.1) |
| Female | 193 (50.3) | 86 (44.8) | 90 (46.9) |
| Body mass index, kg/m2 | 28.2±4.9 | 27.9±4.4 | 28.2±5.3 |
| Society of Thoracic Surgeons (STS) score, % | 2.6 [1.7-4.0] | 2.6 [1.8-4.0] | 2.7 [1.5-4.0] |
| Risk category according to STS score | |||
| Low risk (<4%) | 290 (75.5) | 144 (75.0) | 145 (75.5) |
| Intermediate risk (4-8%) | 78 (20.3) | 39 (20.3) | 39 (20.3) |
| High risk (>8%) | 16 (4.2) | 9 (4.7) | 8 (4.2) |
| Estimated glomerular filtration rate <60 ml/min | 171/362 (47.2) | 85/180 (47.2) | 91/180 (50.6) |
| Estimated glomerular filtration rate <30 ml/min | 53/362 (14.6) | 31/180 (17.2) | 23/180 (12.8) |
| Small annulus: aortic annulus area ≤430 mm2 | 125 (32.6) | 64 (33.3) | 56 (29.2) |
| Bicuspid valve | 23 (6.0) | 14 (7.3) | 15 (7.8) |
| Diabetes | 111 (28.9) | 56 (29.2) | 58 (30.2) |
| Hypercholesterolaemia | 42 (10.9) | 3 (1.6) | 33 (17.2) |
| Hypertension | 256 (66.7) | 129 (67.2) | 125 (65.1) |
| Chronic obstructive pulmonary disease | 42 (10.9) | 20 (10.4) | 20 (10.4) |
| History of atrial fibrillation or flutter | 94 (24.5) | 45 (23.4) | 54 (28.1) |
| Previous stroke | 13 (3.4) | 3 (1.6) | 5 (2.6) |
| Permanent pacemaker | 11 (2.9) | 6 (3.1) | 12 (6.3) |
| Previous MI | 26 (6.8) | 12 (6.3) | 11 (5.7) |
| Previous CABG | 13 (3.4) | 10 (5.2) | 11 (5.7) |
| History of percutaneous coronary intervention | 30 (7.8) | 9 (4.7) | 16 (8.3) |
| History of cerebrovascular accident or a transient ischaemic attack in previous 6 months | 5 (1.3) | 0 (0) | 1 (0.5) |
| Data are presented as n (%), n/N (%), mean±standard deviation, or median [interquartile range]. aBy Meril Life Sciences; bby Edwards Lifesciences; cby Medtronic. CABG: coronary artery bypass graft; MI: myocardial infarction; THV: transcatheter heart valve | |||
Procedural characteristics
The procedural characteristics are tabulated in Table 2.
The mean annular areas were comparable: 470.5±80.0 mm2, 469.3±82.6 mm2 and 473.5±74.2 mm2 in the Myval, SAPIEN and Evolut THV series arms, respectively. Similarly, the mean annular perimeters were 77.8±6.7 mm, 77.7±6.9 mm and 78.1±6.1 mm, respectively. Owing to crossovers, 379 Myval THV series, 189 SAPIEN THV series and 188 Evolut THV series were implanted. In the Myval THV series arm, the Myval THV (91.3%) was the predominant device, followed by the Myval Octacor (8.7%). In the SAPIEN THV series arm, the SAPIEN 3 (55.4%) and the SAPIEN 3 Ultra (44.6%) were implanted, whilst in the Evolut THV series arm, the most implanted device was Evolut PRO (55.2%) followed by Evolut R (37.0%), Evolut PRO+ (5.2%) and Evolut FX (2.6%). All of the patients in the Evolut THV series arm received 26 mm or above devices; no patient received a 23 mm device. The intermediate-size Myval THV series (21.5 mm, 24.5 mm, and 27.5 mm) constituted 48% of the implanted Myvals; these sizes are not available in the SAPIEN or Evolut THV series. Predilation was performed more frequently in the Myval THV series arm as compared to the SAPIEN THV series arm (43.3% vs 30.7%; p=0.005) and equally as compared to the Evolut THV series arm (43.3% vs 45.7%; p=0.64). Post-dilation rates were comparable between the Myval and SAPIEN THV series arms (10.0% vs 10.1%; p=1.00) and were significantly lower with the Myval THV series as compared to the Evolut THV series (10.0% vs 32.5%; p<0.0001).
Table 2. Procedural characteristics of all three cohorts in the LANDMARK trial.
| Procedural details | Myval THV series n=384 | SAPIEN THV series n=192 | Evolut THV series n=192 | p-value Myval vs SAPIEN | p-value Myval vs Evolut |
|---|---|---|---|---|---|
| Access site | |||||
| Transfemoral | 378 (99.7) | 188 (97.5) | 188 (100.0) | 1.00 | 1.00 |
| Right femoral | 334 (88.1) | 167 (88.4) | 168 (89.4) | 0.98 | 0.83 |
| Percutaneous | 297 (78.4) | 158 (83.6) | 141 (75.0) | 0.06 | 0.15 |
| Surgical cutdown | 37 (9.8) | 9 (4.8) | 27 (14.4) | ||
| Left femoral | 44 (11.6) | 21 (11.1) | 20 (10.6) | 0.98 | 0.83 |
| Percutaneous | 40 (10.6) | 20 (10.6) | 18 (9.6) | 1.00 | 1.00 |
| Surgical cutdown | 4 (1.1) | 1 (0.5) | 2 (1.1) | ||
| Subclavian | 1 (0.3) | 1 (0.5) | 0 (0) | 1.00 | 1.00 |
| Right subclavian artery | 0 (0) | 1 (0.5) | 0 (0) | 1.00 | 1.00 |
| Percutaneous | 0 (0) | 0 (0) | 0 (0) | 1.00 | 1.00 |
| Surgical cutdown | 0 (0) | 1 (0.5) | 0 (0) | ||
| Left subclavian artery | 1 (0.3) | 0 (0) | 0 (0) | 1.00 | 1.00 |
| Percutaneous | 0 (0) | 0 (0) | 0 (0) | 1.00 | 1.00 |
| Surgical cutdown | 1 (0.3) | 0 (0) | 0 (0) | ||
| Annular area, mm2 | 470.5±80.0 (n=384) | 469.3±82.6 (n=192) | 473.5±74.2 (n=192) | 0.81 | 0.60 |
| Annular perimeter, mm | 77.8±6.7 (n=384) | 77.7±6.9 (n=192) | 78.1±6.1 (n=192) | 0.72 | 0.60 |
| Procedural time, min | 77.0±40.3 (n=378) | 76.5±43.2 (n=189) | 78.7±37.1 (n=188) | 0.63 | 0.31 |
| Contrast volume, ml | 143.6±68.5 (n=355) | 144.9±65.6 (n=189) | 155.2±79.1 (n=175) | 0.64 | 0.21 |
| General anaesthesia | 73 (19.3) (n=379) | 24 (12.7)(n=189) | 50 (26.6) (n=188) | 0.07 | 0.06 |
| Conscious sedation | 306 (80.7) (n=379) | 165 (87.3) (n=189) | 138 (73.4) (n=188) | ||
| Predilation | 164 (43.3) (n=379) | 58 (30.7) (n=189) | 86 (45.7) (n=188) | 0.005 | 0.64 |
| TAVI device implanted | 379 | 189 | 188 | ||
RF after implantation, prior to post-dilation, % |
12.0 (6.0, 18.5) (n=23) | 18.0 (1.0, 19.0) (n=9) | 10.5 (6.0, 15.0) (n=26) | 0.79 | 0.62 |
RF >17% after implantation, prior to post-dilation |
6 (26.1) (n=23) | 5 (55.6)(n=9) | 6 (23.1) (n=26) | 0.21 | 1.00 |
| Post-dilation | 38 (10.0) (n=379) | 19 (10.1) (n=189) | 61 (32.5) (n=188) | 1.00 | <0.0001 |
| RF after post-dilation, % | 2.0 (1.0, 8.0) (n=33) | 3.0 (2.0, 8.0) (n=17) | 5.0 (1.0, 9.5) (n=47) | 0.37 | 0.21 |
| RF >17% after post-dilation | 0 (0) (n=33) | 1 (5.9) (n=17) | 4 (8.5) (n=47) | 0.34 | 0.14 |
| RF in final aortogram, % | 3.0 (1.0, 7.0) (n=295) | 3.0 (1.0, 7.0) (n=151) | 5.0 (1.0, 10.0) (n=150) | 0.86 | 0.0007 |
| RF >17% | 6 (2.0)(n=295) | 6 (4.0) n=(151) | 12 (8.0) n=(150) | 0.23 | 0.006 |
| Cerebral protection device | 48 (13.2) | 12 (6.8) | 21 (11.4) | 0.03 | 0.71 |
| Use of closure device | 344 (90.8) | 180 (95.2) | 160 (85.1) | 0.09 | 0.06 |
| Length of hospital stay, days | 4.0 (3.0, 6.0) (n=374) | 4.0 (2.0, 6.0) (n=189) | 4.0 (2.0, 6.0)(n=186) | 0.72 | 0.66 |
| Data are presented as n, n (%), mean±standard deviation, or median (Q1, Q3). Q: quartile; RF: regurgitation fraction assessed by videodensitometry of the aortography; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve | |||||
Primary outcome
The probability distribution (with point estimate and 1-sided 95% CI) of the risk difference for the frequency of the primary endpoint between the Myval versus SAPIEN or Evolut THV series arm is depicted in Figure 2. The itemised primary outcomes of all three arms are shown in Figure 3 and Table 3.
At 30 days, the primary composite endpoint (non-inferiority analysis) occurred in 24.7% in the Myval THV series arm, 24.1% in the SAPIEN THV series arm (absolute risk difference: 0.6%, with the 1-sided upper 95% CI limit of 8.0%) and 30.0% in the Evolut THV series arm (absolute risk difference: –5.3%, with the 1-sided upper 95% CI limit of 2.5%). Therefore, as the predefined non-inferiority margin was 10.44%, the Myval THV series achieved statistically significant non-inferiority compared with the SAPIEN THV series (pnon-inferiority=0.0033) and with the Evolut THV series (pnon-inferiority <0.0001). Secondary analyses of the components of the primary endpoint showed no significant differences between the Myval versus SAPIEN THV series arms or Myval versus Evolut THV series arms (Table 3). Of note, the p-value for the risk difference between the SAPIEN and Myval THV series for bleeding was 0.07 in favour of the SAPIEN THV series (0.5% vs 2.9%) and between the Myval and Evolut THV series, PVR was 0.06 in favour of the Myval THV series (3.4% vs 7.4%). There were no other trends in risk difference among types of valve for events such as mortality, stroke, PPI, acute kidney injury or major vascular complications.
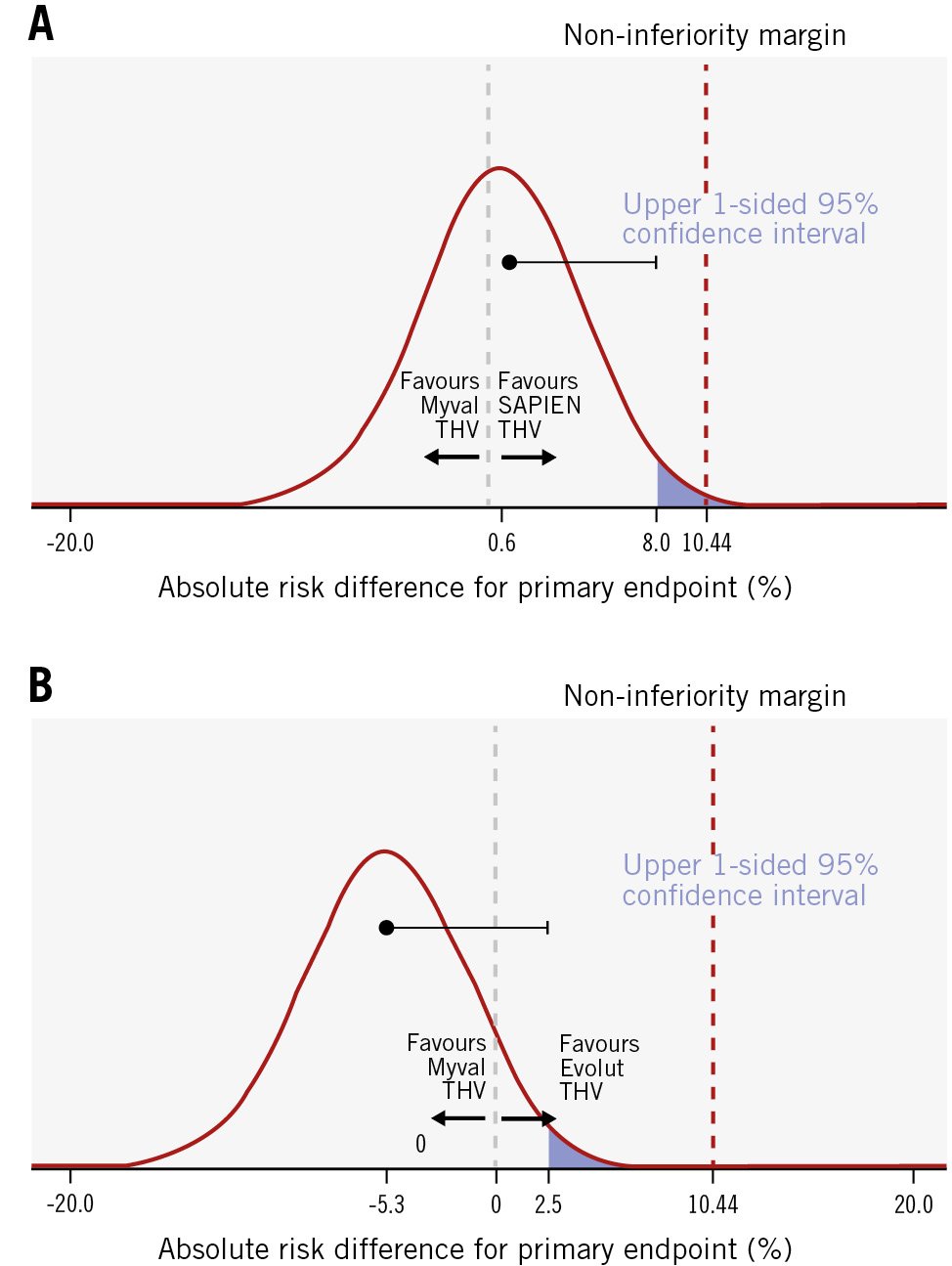
Figure 2. Probability distribution (with point estimate and 1-sided 95% CI based on the Farrington-Manning test) of the risk difference for the frequency of the primary endpoint. The risk difference is provided for the Myval THV series arm versus the SAPIEN THV series arm (A) and the Myval THV series arm versus the Evolut THV series arm (B). CI: confidence interval; THV: transcatheter heart valve
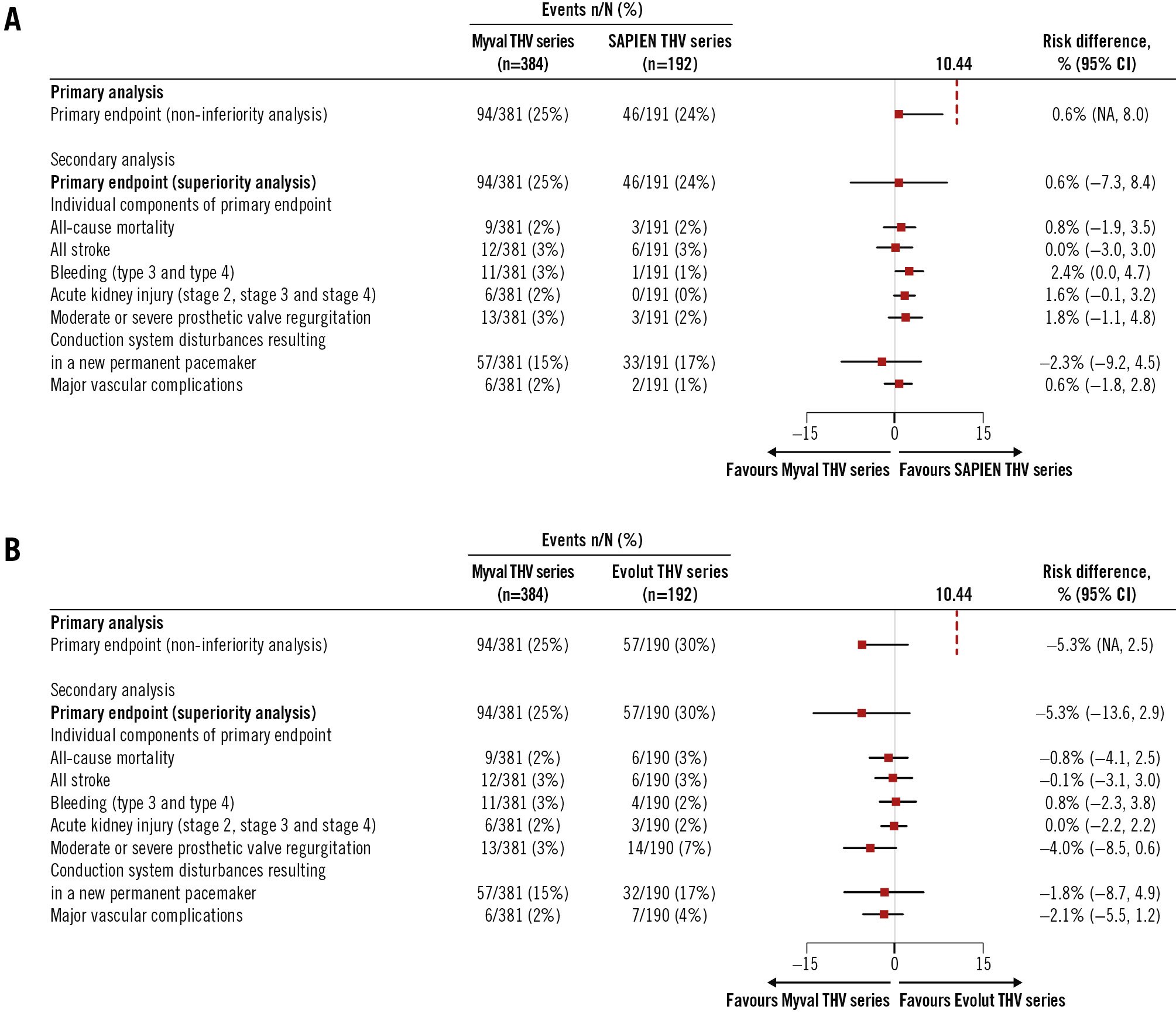
Figure 3. Primary and secondary analyses of the primary endpoint and its components in the intention-to-treat population. A) Myval THV series arm versus SAPIEN THV series arm; B) Myval THV series arm versus Evolut THV series arm. CI: confidence interval; NA: not applicable; THV: transcatheter heart valve
Table 3. Primary outcomes of all three cohorts in the LANDMARK trial.
| Events | Myval THV series n=384 | SAPIEN THV series n=192 | Evolut THV valves n=192 | Risk difference* Myval vs SAPIEN | p-value Myval vs SAPIEN | Risk difference* Myval vs Evolut | p-value Myval vs Evolut |
|---|---|---|---|---|---|---|---|
| Primary analysis | |||||||
| Primary endpoint (non-inferiority analysis) | 94/381 (24.7) | 46/191 (24.1) | 57/190 (30.0) | 0.6(NA to 8.0) | 0.0033 | –5.3(NA to 2.5) | <0.0001 |
| Secondary analysis | |||||||
| Primary endpoint (superiority analysis) | 94/381 (24.7) | 46/191 (24.1) | 57/190 (30.0) | 0.6(–7.3 to 8.4) | 0.96 | –5.3(–13.6 to 2.9) | 0.21 |
| Individual components of the primary endpoint | |||||||
| All-cause mortality | 9/381 (2.4) | 3/191 (1.6) | 6/190 (3.2) | 0.8(–1.9 to 3.5) | 0.76 | –0.8(–4.1 to 2.5) | 0.59 |
| All stroke | 12/381 (3.1) | 6/191 (3.1) | 6/190 (3.2) | 0.0(–3.0 to 3.0) | 1.00 | –0.1(–3.1 to 3.0) | 1.00 |
| Bleeding (type 3 and type 4) | 11/381 (2.9) | 1/191 (0.5) | 4/190 (2.1) | 2.4(0.0 to 4.7) | 0.07 | 0.8(–2.3 to 3.8) | 0.78 |
| Acute kidney injury (stage 2, stage 3 and stage 4) | 6/381 (1.6) | 0/191(0) | 3/190 (1.6) | 1.6(–0.1 to 3.2) | 0.19 | 0.0(–2.2 to 2.2) | 1.00 |
| Moderate or severe prosthetic valve regurgitation | 13/381 (3.4) | 3/191 (1.6) | 14/190 (7.4) | 1.8(–1.1 to 4.8) | 0.32 | –4.0(–8.5 to 0.6) | 0.06 |
| Conduction system disturbances resulting in a new permanent pacemaker | 57/381 (15.0) | 33/191 (17.3) | 32/190 (16.8) | –2.3(–9.2 to 4.5) | 0.55 | –1.8(–8.7 to 4.9) | 0.64 |
| Major vascular complications | 6/381 (1.6) | 2/191 (1.0) | 7/190 (3.7) | 0.6(–1.8 to 2.8) | 0.72 | –2.1(–5.5 to 1.2) | 0.14 |
| Data are n/N (%) or risk difference (95% confidence interval). *All 95% confidence intervals and p-values are two-sided except those of the primary composite endpoint analysis for non-inferiority (one-sided). THV: transcatheter heart valve | |||||||
Secondary outcomes
Technical success and device success
Technical and device success rates are shown in Supplementary Table 1. Technical success rates at the end of the procedure were 96.3%, 98.9%, and 94.7% in the Myval, SAPIEN, and Evolut THV series arms, respectively. At 30-day follow-up, the device success rates were 91.0%, 92.6%, and 86.7%, respectively.
Conduction disturbances and PPI rates
The rates of PPI in the Myval, SAPIEN and Evolut THV series arms were 15.0%, 17.3% and 16.8%, respectively, with the underlying indications reported in Supplementary Table 2. The new-onset left bundle branch block (LBBB) rates were comparable (Myval THV series: 11.5% [n=39/339], SAPIEN THV series: 10.0% [n=17/170], Evolut THV series: 14.3% [n=23/161]): pMyval-SAPIEN=0.72 and pMyval-Evolut=0.46.
Haemodynamic parameters
Echocardiographic assessment of the three arms are shown in Table 4. Rates of severe patient-prosthesis mismatch, based on body mass index, were comparable in the Myval, SAPIEN and Evolut THV series arms at 4.0% (n=15/372), 5.9% (n=11/188) and 1.7% (n=3/179), respectively (pMyval-SAPIEN=0.45 and pMyval-Evolut=0.23). The rates of moderate-severe PVR were also similar (3.4% vs 1.6% vs 7.4%) (Table 3).
At 30 days, the Myval THV series had a significantly lower aortic valve mean pressure gradient (MPG) (8.2±3.5 mmHg vs 10.2±4.9 mmHg; p<0.0001) and higher effective orifice area (EOA) (2.02±0.55 cm2 vs 1.80±0.52 cm2; p<0.0001) compared to the SAPIEN THV series (Table 4), whereas it had a significantly higher MPG (8.2±3.5 mmHg vs 5.6±2.3 mmHg; p<0.0001) and lower EOA (2.02±0.55 cm2 vs 2.31±0.55 cm2; p<0.0001) compared to the Evolut THV series (Table 4). A THV size-specific comparison of the mean EOA of the 23 mm (1.80±0.49 cmm2 vs 1.58±0.49 cm2; p=0.01), 26 mm (2.13±0.52 cm2 vs 1.90±0.45 cm2; p=0.0041) and 29 mm (2.43±0.61 cm2 vs 2.05±0.48 cm2; p=0.01) Myval and SAPIEN valves showed that the mean EOA with the Myval THV series was significantly larger than with the SAPIEN THV series. The mean EOAs of the 20 mm nominal sizes of the Myval and SAPIEN THV series (1.42±0.05 cm2 vs 1.38±0.36 cm2; p=0.78) were comparable (Figure 4). A similar THV size-specific comparison of the mean EOAs of the 26 mm (2.13±0.52 cm2 vs 2.22±0.44 cm2; p=1.00) and 29 mm (2.43±0.61 cm2 vs 2.27±0.52 cm2; p=0.48) Myval and Evolut THV series showed no significant difference (Figure 4). None of the patients were implanted with a 23 mm Evolut valve and, per design and protocol, the 30.5 mm and 32 mm Myval THV series were not included in the randomised trial and thus not available for comparison with the 34 mm Evolut THV series.
In terms of RF% assessed by quantitative aortography on the final angiogram, the RF (median 3.0% [1st, 3rd quartiles: 1.0, 7.0]) was comparable between the Myval and SAPIEN THV series, whilst the difference between the Myval and Evolut THV series (3.0% [1.0, 7.0] and 5.0% [1.0, 10.0]; p=0.0007) was highly significant. An RF higher than 17% was documented in 2%, 4% and 8% of the patients in the Myval, SAPIEN (pMyval-SAPIEN=0.23) and Evolut (pMyval-Evolut=0.0057) THV series arms, respectively (Table 2).
Table 4. Echocardiographic data for Myval versus SAPIEN THV series and Myval versus Evolut THV series.
| Myval vs SAPIEN THV series | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Baseline | Discharge | 30 days | p-value (baseline vs 30 days) | |||||||
| Myval THV series | SAPIEN THV series | p-value | Myval THV series | SAPIEN THV series | p-value | Myval THV series | SAPIEN THV series | p-value | Myval THV series | SAPIEN THV series | |
| Effective orifice area, cm2 | 0.74±0.22(n=364) | 0.70±0.21(n=180) | 0.04 | 2.16±0.61(n=353) | 1.84±0.51(n=175) | <0.0001 | 2.02±0.55(n=346) | 1.80±0.52(n=169) | <0.0001 | <0.0001 | <0.0001 |
| AV mean pressure gradient, mmHg | 39.9±14.0 (n=368) | 39.2±14.2(n=184) | 0.57 | 8.3±4.0(n=362) | 10.9±4.6(n=181) | <0.0001 | 8.2±3.5(n=355) | 10.2±4.9(n= 174) | <0.0001 | <0.0001 | <0.0001 |
| Aortic regurgitation assessment | n=360 | n=186 | n=362 | n=184 | n=350 | n=171 | |||||
| None/trace-mild | 318 (88.3) | 165 (88.7) | 1.00 | 351 (97.0) | 181 (98.4) | 0.40 | 341 (97.4) | 168 (98.3) | 0.76 | <0.0001 | 0.0007 |
| Moderate-severe | 42 (11.7) | 21 (11.3) | 1.00 | 11 (3.0) | 3 (1.6) | 0.40 | 9 (2.6) | 3 (1.8) | 0.76 | <0.0001 | 0.0007 |
| Myval vs Evolut THV series | |||||||||||
| Parameter | Baseline | Discharge | 30 days | p-value (baseline vs 30 days) | |||||||
| Myval THV series | Evolut THV series | p-value | Myval THV series | Evolut THV series | p-value | Myval THV series | Evolut THV series | p-value | Myval THV series | Evolut THV series | |
| Effective orifice area, cm2 | 0.74±0.22 (n=364) | 0.74±0.23 (n=180) | 0.91 | 2.16±0.61 (n=353) | 2.35±0.56 (n=171) | 0.0003 | 2.02±0.55 (n=346) | 2.31±0.55(n=168) | <0.0001 | <0.0001 | <0.0001 |
| AV mean pressure gradient, mmHg | 39.9±14.0 (n=368) | 38.2±12.9 (n=184) | 0.16 | 8.3±4.0(n=362) | 5.9±2.5 (n=175) | <0.0001 | 8.2±3.5(n=355) | 5.6±2.3 (n=175) | <0.0001 | <0.0001 | <0.0001 |
| Aortic regurgitation assessment | n=360 | n=182 | n=362 | n=173 | n=350 | n=174 | |||||
| None/trace-mild | 318 (88.3) | 156 (85.7) | 0.46 | 351 (97.0) | 161 (93.1) | 0.06 | 341 (97.4) | 163 (93.7) | 0.06 | <0.0001 | 0.02 |
| Moderate-severe | 42 (11.7) | 26 (14.3) | 0.46 | 11 (3.0) | 12 (6.9) | 0.06 | 9 (2.6) | 11 (6.3) | 0.06 | <0.0001 | 0.02 |
| Data are presented as n (%) or mean±standard deviation. AV: aortic valve; THV: transcatheter heart valve | |||||||||||
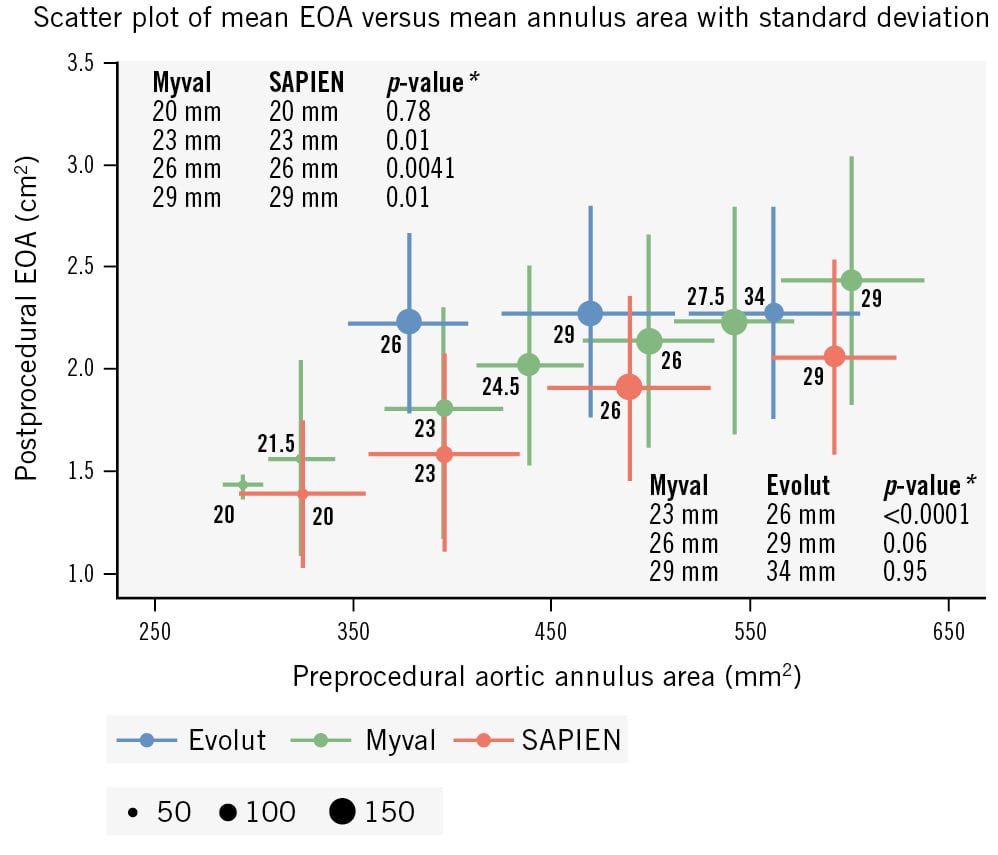
Figure 4. Scatter plot of postprocedural EOA (cm2) as assessed by echocardiography, and preprocedural aortic annulus area (mm2) as assessed by computed tomography, categorised according to the nominal size of the three different valve series. *P-values are based on 2-sample t-test. EOA: effective orifice area
Quality of life
In all three arms, there were significant (p<0.0001) improvements in the NYHA Functional Class (Supplementary Figure 1), distance covered in the six-minute walk test (Supplementary Table 3), and physical and mental quality-of-life scores (Supplementary Table 4) between baseline and 30-day follow-up; however, no differences were noted between the Myval versus SAPIEN THV series and the Myval versus Evolut THV series.
Patients with a small aortic annulus
The clinical and echocardiographic outcomes in patients with a small aortic annulus (≤430 mm²) are shown in Supplementary Table 5 and Table 5. The event rates of the primary composite endpoint were comparable between the three arms (Myval THV series: 20%, SAPIEN THV series: 21% and Evolut THV series: 33%; pMyval-SAPIEN=1.00 and pMyval-Evolut=0.08) (Supplementary Table 5). The mean EOA was significantly larger in the Evolut THV series (2.27±0.49 cm2) arm than in the Myval THV series arm (1.75±0.49 cm2) or SAPIEN THV series arm (1.53±0.45 cm2) (pMyval-SAPIEN=0.006 and pMyval-Evolut<0.0001). The MPG was significantly lower in the Myval THV series arm compared to the SAPIEN THV series arm (9.30±3.74 mmHg vs 11.78±5.40 mmHg; p=0.0005), and the Evolut THV series arm had a significantly lower MPG than the Myval THV series arm (5.76±2.33 mmHg; p<0.0001) (Table 5).
Table 5. Echocardiographic data in patients with a small aortic annulus (≤430 mm2).
| Parameters | Baseline | Discharge | 30-day follow-up | p-value (at 30 days) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Myval THV series | SAPIEN THV series | Evolut THV series | Myval THV series | SAPIEN THV series | Evolut THV series | Myval THV series | SAPIEN THV series | Evolut THV series | Myval vs SAPIEN | Myval vs Evolut | |
| Effective orifice area, cm2 | 0.70±0.20(n=118) | 0.65±0.19(n=60) | 0.72±0.24(n=52) | 1.86±0.55(n=113) | 1.62±0.41(n=60) | 2.28±0.52(n=48) | 1.75±0.49(n=112) | 1.53±0.45(n=58) | 2.27±0.49(n=47) | 0.006 | <0.0001 |
| AV mean pressure gradient, mmHg | 41.05±13.52(n=119) | 42.53±14.92(n=60) | 39.88±14.37(n=54) | 9.48±4.88(n=116) | 12.73±4.89(n=62) | 6.14±2.76(n=49) | 9.30±3.74(n=116) | 11.78±5.40(n=59) | 5.76±2.33(n=50) | 0.0005 | <0.0001 |
| Aortic regurgitation grade | n=112 | n=61 | n=53 | n=115 | n=61 | n=51 | n=109 | n=58 | n=52 | - | - |
| None or trace | 39 (35) | 18 (30) | 14 (26) | 81 (70) | 52 (85) | 20 (39) | 74 (68) | 47 (81) | 23 (44) | 0.03 | 0.0003 |
| Mild | 58 (52) | 28 (46) | 31 (58) | 31 (27) | 8 (13) | 24 (47) | 35 (32) | 10 (17) | 23 (44) | ||
| Moderate | 9 (8) | 14 (23) | 8 (15) | 1 (1) | 0 (0) | 5 (10) | 0 (0) | 0 (0) | 5 (10) | ||
| Severe | 2 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Not evaluable | 4 (4) | 1 (2) | 0 (0) | 2 (2) | 1 (2) | 2 (4) | 0 (0) | 1 (2) | 1 (2) | ||
| Data are presented as mean±standard deviation or n (%). AV: aortic valve; THV: transcatheter heart valve | |||||||||||
Discussion
Composite clinical primary endpoints among the three valves
This substudy of the LANDMARK trial compared outcomes for the first time between two BEVs – the Myval THV series and the SAPIEN THV series – in addition to comparing the Myval THV series to the SE Evolut THV series. The key findings of this substudy are the individual non-inferiority of the Myval THV series to the SAPIEN THV series and to the Evolut THV series for the primary composite safety and effectiveness endpoint at 30-day follow-up. Additionally, no significant differences were found between the arms for any components of the primary composite endpoint.
Haemodynamic assessment among the three valves
At 30 days, the MPG (p<0.0001) and EOA (p<0.0001) were significantly better with the Myval THV series than the SAPIEN THV series and with the Evolut THV series than the Myval THV series.
Notably, there were no significant differences in EOA between the Myval and Evolut THV series for the 26 mm (2.13±0.52 cm2 vs 2.22±0.44 cm2; p=1.00) or 29 mm (2.43±0.61 cm2 vs 2.27±0.52 cm2; p=0.48) diameter valves (Figure 4). The overall better haemodynamics of the SE Evolut THV series in the LANDMARK trial is mainly due to the more favourable EOA and gradients observed in the patients with a small annulus who were exclusively treated with a 26 mm THV, instead of a 23 mm THV. These findings align with the SMART trial, which also used a minimal (2.3%) number of 23 mm Evolut THVs9. As a matter of fact, EOA and gradients in all prior studies have been reported based on echocardiographic assessment. Although echocardiographic assessments generally report better haemodynamics for supra-annular SE valves (SEVs), controversy remains about their durability compared to BEVs, suggesting a need for invasive gradient analysis10. Only one case in the Myval THV series arm received a 30.5 mm THV (protocol deviation) with a resultant EOA of 3.30 cm2, whereas in the Evolut 34 mm group (n=44), the average EOA was 2.42±0.65 cm2, similar to the Myval 29 mm group (2.43±0.61 cm2; p=0.95).
In patients with a small aortic annulus, the mean EOAs were significantly larger in the Evolut THV series arm than in the Myval and SAPIEN THV series arms. In a recent meta-analysis of 21 studies (n=8,647) comparing SEVs and BEVs in small aortic annuli, SEVs had superior haemodynamics, but higher rates of paravalvular leak, PPI and in-hospital stroke11. The significantly larger EOA of the Evolut THV series in patients with a small aortic annulus may represent a drawback in the LANDMARK trial: there were seven instances of PVR (7/59, 11.9%) in the Evolut THV series arm compared to two (2/117, 1.7%; p=0.007) in the Myval THV series arm (Supplementary Figure 2). The observed relatively high PVR rate with the Evolut THV series in small annulus patients may be related to non-uniform expansion of the SEV. Moscarelli et al reported that non-uniform expansion is consistently observed after implantation of a SEV, with eccentricity more frequent at the annular level compared to the prosthesis frame outflow level12. It has also been demonstrated that underexpansion and non-uniform expansion of the SEV could result in an elliptical shape of the stent frame at the level of leaflet coaptation, which is associated with an increased incidence of PVR and putatively resulted in a pinwheeling effect that could generate strain on the leaflet and affect a THV’s long-term durability13.
The overall comparison of moderate/severe PVR between the Myval and Evolut (including Evolut R) THV series showed a p-value of 0.06 (Myval THV series: 3.4% vs Evolut THV series: 7.4%; risk difference: –4.0%, 95% CI: –8.5 to 0.6). However, a sensitivity analysis for moderate-severe PVR between the Myval and Evolut THV series, after excluding the Evolut R, showed a p-value of 0.28 (Myval THV series: 3.4% vs Evolut THV series: 6.1%; risk difference: –2.68%, 95% CI: –7.98 to 2.63) (Supplementary Table 6).
The incidence of moderate-severe PVR in the LANDMARK trial is similar to in the SCOPE I and SOLVE-TAVI trials, showing that BEVs have lower rates than SEVs1415. Balloon post-dilation (BPD) is a commonly used technique for minimising the degree of PVR following TAVI16. However, it is associated with serious complications such as annular rupture, stroke, and damage to the prosthetic leaflets, which may increase the risk of early THV deterioration17. A significantly lower proportion of patients required BPD in the BE Myval and SAPIEN THV series arms (10.0% and 10.1%) compared to the SE Evolut THV series arm (32.5%; p<0.0001).
Notably, contemporary THV designs with better sealing skirts have gradually reduced the frequency of more-than-mild PVR1819. The lower rates of PVR with the Myval THV series could be due to the internal and external skirt design (reduces PVR) and the availability of intermediate sizes, which eliminates the need for over- and undersizing and results in an ideal fit to the native annulus.
A key innovation of the Myval THV series is its availability of intermediate sizes with 1.5 mm differences, compared to the conventional 3 mm step-up in nominal sizes. Our study found that about half of the Myval patients were implanted with these intermediate sizes based on preprocedural MSCT assessments, potentially preventing oversizing or undersizing. The Myval THV series shows a higher and narrower density curve of fitting index compared to the SAPIEN THV series (Supplementary Figure 3). More patients implanted with a Myval THV series had a fitting index (the ratio between the nominal THV diameter and MSCT-derived aortic annulus diameter) around 1.0, indicating proper fit, which can be attributed to the wider range of sizes. This better sizing and fitting may contribute to the superior EOA and lower transvalvular gradient in the Myval THV series (Supplementary Table 7) and may help reduce the occurrence of PVR and PPI20. With appropriate fitting, PPI rates were similar between the two valves (15.0% vs 14.1%), but there was a numerical difference favouring the Myval THV series (15.2% vs 21.1%) when fitting was more appropriate, possibly due to the use of intermediate sizes. A future pooled analysis of the LANDMARK and Compare-TAVI trials may validate these hypotheses.
Occurrence of PPI among the three valves
New PPI rates were comparable among the three arms (Myval THV series: 15.0%, SAPIEN THV series: 17.3%, Evolut THV series: 16.8%). Previous reports found that SEV implantation is a predictor for new PPI after TAVI, next to age, baseline right bundle branch block (RBBB), baseline LBBB and THV implantation depth21.
A recent meta-analysis of 23 studies (n=18,610) reported a crude incidence of 17% for PPI (range 8.8% to 32%), consistent with our results. However, SEVs and baseline RBBB were associated with a 2-fold greater risk of continued pacemaker dependency 1 year after TAVI22.
The cusp-overlap view technique for SEVs and a high deployment technique for BEVs and SEVs have proven helpful in reducing conduction abnormalities and new PPI2324. The Myval THV series shows less shortening (Myval Octacor: 19-20%, Myval THV series: 21-24%, SAPIEN THV series: 26-27%, Evolut THV series: 44%), facilitates precision placement and deployment accuracy, and may reduce PVR and PPI requirement25. Again, these remain hypotheses until outcomes from the pooled analysis (>1,500 patients) from LANDMARK and Compare-TAVI are available.
Technological improvements with new THV devices and overcoming the learning curve could also help to reduce the need for PPI. It is crucial to analyse each baseline ECG for future PPI risk and to assess the patient’s anatomy before TAVI, as patients with a shorter membranous septum may benefit from the MInimizing Depth According to the membranous Septum (MIDAS) technique26. However, it is equally important to recognise that, in 23% of patients, the membranous septum terminates above the annular plane, leaving the operator no room to manoeuvre27.
Limitations
Our study has a few limitations. First, multiple THV iterations were used across all arms, limiting representation of the latest devices, which were unavailable in Europe during enrolment (e.g., SAPIEN Resilia). Second, the decision for new PPI was left to the investigator’s discretion, potentially introducing bias. Third, the study evaluated only early 30-day outcomes; long-term outcomes are essential for robust device comparison. For the following 10 years, clinical and echocardiographic evaluations will be carried out together with ongoing data monitoring for long-term analyses. While the current findings are encouraging, they must be verified in long-term follow-up. The superiority in haemodynamic performance of SEVs compared to BEVs in small aortic annulus patients needs further investigation to determine if these short-term benefits are sustained clinically, haemodynamically, and in terms of durability.
Conclusions
In conclusion, this prespecified substudy of the LANDMARK trial demonstrated that the Myval THV series was non-inferior to the SAPIEN THV series and the Evolut THV series in terms of early safety and effectiveness at 30 days in elderly patients with severe, symptomatic aortic stenosis.
Impact on daily practice
The LANDMARK trial demonstrates that the Myval transcatheter heart valve (THV) series is non-inferior to both the SAPIEN and Evolut THV series in terms of safety and effectiveness at 30 days. One-year outcomes of the LANDMARK trial as well as the Compare-TAVI trial (Myval vs SAPIEN) will be available soon and are eagerly awaited.
Funding
This trial was funded by Meril Life Sciences. The funder provided all financial support to conduct this trial. The trial protocol, the data analysis and the writing of the manuscript were supervised by the steering committee in consultation with the funder.
Conflict of interest statement
N. van Royen reports grant funding and personal fees from Abbott; grants from Philips, Biotronik, and Medtronic; speaker fees from MicroPort, Bayer, and RainMed Medical outside the submitted work; and travel support to attend meeting from Meril Life Sciences. I.J. Amat-Santos reports being a proctor for Medtronic, Boston Scientific, and Meril Life Sciences. A. Ijsselmuiden reports consulting fees from Meril Life Sciences, Angiocare, PulseCath, and Cardiawave; received an institutional grant from Medtronic and Abbott. D. Unic reports payment for workshops from Medtronic; and is a member of the Medtronic EMEA surgical advisory board. B. Merkely reports institutional grants and speaker fees from Abbott, AstraZeneca, Biotronik, Boehringer Ingelheim, CSL Behring, Daiichi Sankyo, DUKE Clinical Institute, Medtronic, and Novartis; institutional fees from Boston Scientific, Bristol-Myers Squibb, Eli Lilly, Terumo, and VIFOR Pharma. R.S. Hermanides reports speaker fees from Amgen, Novartis, Edwards Lifesciences, Meril Life Sciences, and Abbott outside the submitted work. P. Martin reports a proctorship grant from Meril Life Sciences; and an educational event grant from Medtronic. E. van Belle is president of the French Interventional Working Group (GACI) and a Board member of EAPCI. A. Linke received grants from Edward Lifesciences and Novartis; speaker honoraria from Edwards Lifesciences, Abiomed, Abbott, Boston Scientific, Novartis, Pfizer, BMS, Daiichi Sankyo, AstraZeneca, Boehringer Ingelheim, Meril Life Sciences, and Corvia; travel support from AstraZeneca, Abbott, Meril Life Sciences, Abiomed and Boston Scientific; is a partial patent holder with Boston Scientific; is a stock option holder with Pi-Cardia, Transverse Medical and Filterlex. K. Toutouzas reports proctorship with Abbott, Meril Life Sciences and Medtronic; consulting fees from Gore Medical; is a Board member of the Hellenic Society of Cardiology. M. De Sousa Almeida reports lecture fees from Medtronic and Novartis; travel support from Medtronic, Terumo and Boston Scientific. F. Bedogni reports grants, consulting fees, payment/honoraria/speaker fees and travel support to attend meetings from Medtronic, Abbott, Boston Scientific and Meril Life Sciences; and reports participation in a DSMB for Abbott. M. Pan reports lecture fees from Medtronic and Abbott. O. Angerås reports proctorship and speaker fees with Meril Life Sciences and Abbott; speaker fees from Medtronic; and support for attending meetings from Meril Life Sciences. W.-K. Kim reports honoraria or consultancy fees from Edwards Lifesciences; consulting fees from Boston Scientific, Meril Life Sciences and Abbott; and participation on data and safety monitoring board for HID Imaging and P&F. J. Rothe reports personal fees for consulting/proctoring from Meril Life Sciences, Medtronic, and Qatna; and travel support for attending meetings from Meril Life Sciences, Edwards Lifesciences, Abbott, Medtronic, and Boston Scientific. D Westermann reports personal fees from Abiomed, AstraZeneca, Boehringer Ingelheim, Novartis, Meril Life Sciences and Medtronic. A. Tobe reports a grant from the Fukuda Foundation for Medical Technology. S. Garg reports honoraria or consultancy fees from Biosensors. U. Chandra and A. Thakkar are full employees of Meril Life Sciences. M.-C. Morice reports that she is shareholder and CEO of CERC, a CRO involved in the trial; and minor shareholder of Electroducer. O. Soliman reports research grants from Biosensors, Boston Scientific, Cardiawave and Meril Life Sciences. P.W. Serruys reports consultancy fees from SMT, Novartis, Meril Life Sciences, and Philips. A. Baumbach reports consultation and speaker fees from AstraZeneca, Sinomed, MicroPort, Medtronic, Faraday, Pi-Cardia, Biosensors, JenaValve and Meril Life Sciences. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

