
Abstract
Aims: The aim of this study was to evaluate 30-day safety and efficacy outcomes of transcatheter aortic valve implantation (TAVI) performed with the SAPIEN 3 Ultra system.
Methods and results: The S3U registry is a physician-led, post-approval, multicentre, observational registry of transfemoral TAVI with the SAPIEN 3 Ultra. New features include an improved sealing skirt, a 14 Fr expandable sheath and a new delivery catheter. Overall, 139 consecutive patients at nine participating centres were enrolled. Mean age was 81.4±8.3 years, average STS score 3.8±2.4%. The vast majority (97.2%) underwent TAVI with local anaesthesia (28.8%) or conscious sedation (68.3%). Balloon predilatation was performed in 30 patients (21.6%), post-dilatation in three (2.2%). In-hospital, there were no cases of death, stroke, or conversion to open heart surgery. Major vascular complications occurred in three patients (2.2%), as well as major or life-threatening bleedings in three patients (2.2%). There were two moderate (1.4%) and no moderate/severe paravalvular leaks. Median length of stay after TAVI was three days (IQR 3-5 days). At 30 days, there were no deaths, MI, or strokes, and the incidence of new permanent pacemaker implantation was 4.4%.
Conclusions: This first multicentre international experience of transfemoral TAVI with the SAPIEN 3 Ultra transcatheter heart valve shows good in-hospital and 30-day clinical outcomes.
Introduction
Transcatheter aortic valve implantation (TAVI) represents an alternative to surgical aortic valve replacement (SAVR) for elderly patients with severe aortic stenosis (AS). Randomised clinical trials have demonstrated the superiority of TAVI over medical therapy in patients at prohibitive surgical risk, and equivalence or superiority over SAVR for all other surgical risk categories1,2,3,4,5,6,7. The field of TAVI is rapidly evolving, with major refinements in technology, procedural techniques, patient selection and biomedical engineering. With the development of improved devices, new approaches and new implantation strategies, TAVI has become much simpler and safer. The first transcatheter heart valve device implanted in man in 2002 was a balloon-expandable device8. Since then, three further device iterations have been released and approved for clinical use by Edwards Lifesciences (Irvine, CA, USA): the SAPIEN9, the SAPIEN XT10, and the SAPIEN 311. Changes have included innovations in the valve, the delivery system and the introducer sheath. The recently released SAPIEN 3 Ultra (Edwards Lifesciences) is the latest development whose features include an improved sealing skirt, a lower profile, and a simplified delivery catheter; it has gained approval for commercial use in Europe and the USA. To date, there have been no reports documenting clinical experience with the SAPIEN 3 Ultra.
We herein report the 30-day outcomes of the S3U registry, a physician-led, post-approval multicentre, real-world observational registry of transfemoral TAVI with the SAPIEN 3 Ultra device.
Methods
STUDY DESIGN AND PATIENT POPULATION
The SAPIEN 3 Ultra received CE mark approval in November 2018. Since then, an initial product evaluation (IPE) phase has been launched, with a limited product release in selected centres across Europe. The IPE was terminated in December 2018. Concurrently, we designed a physician-led, international, multicentre, prospective registry aimed at collecting clinical, echocardiographic, procedural and outcome data of the first consecutive transfemoral SAPIEN 3 Ultra procedures performed within the IPE phase and up to February 2019. In total, nine centres participated in the study. The indication for TAVI was determined by a Heart Team. The selection of the SAPIEN 3 Ultra was based on labelled indications and the final decision was then left to the operators. The manufacturer of the SAPIEN 3 Ultra, Edwards Lifesciences, had no role in data collection, analysis, or manuscript drafting and did not provide any financial support for the study.
THE SAPIEN 3 ULTRA DEVICE (Figure 1)
The SAPIEN 3 Ultra retains the SAPIEN 3 cobalt-chromium alloy frame, with a low delivery profile and high radial strength. The SAPIEN 3 Ultra characteristics and differences compared to the SAPIEN 3 are shown in Figure 1. Briefly, the most important changes are related to the outer skirt made from a textured polyethylene terephthalate (PET) and 40% higher than that of the SAPIEN 3 for a better final sealing. The SAPIEN 3 Ultra is available in three sizes (20, 23 and 26 mm), whereas the 29 mm valve is the SAPIEN 3 mounted on the Ultra delivery system. The delivery system was also modified with on-balloon valve crimping to streamline the procedure, eliminating the need for valve alignment and flex catheter retraction steps. The crossing profile is lower thanks to smooth tip-to-valve transition and to a shorter tapered distal tip. Finally, the Axela sheath has replaced the eSheath with new features. It is a 14 Fr expandable sheath for all valve sizes, including the 29 mm SAPIEN 3. It has a hydrophilic coating for smooth insertion, tracking and removal. The specific design allows transient expansion and active contraction and, by means of a seamless design, it maintains optimal haemostasis throughout the procedure.
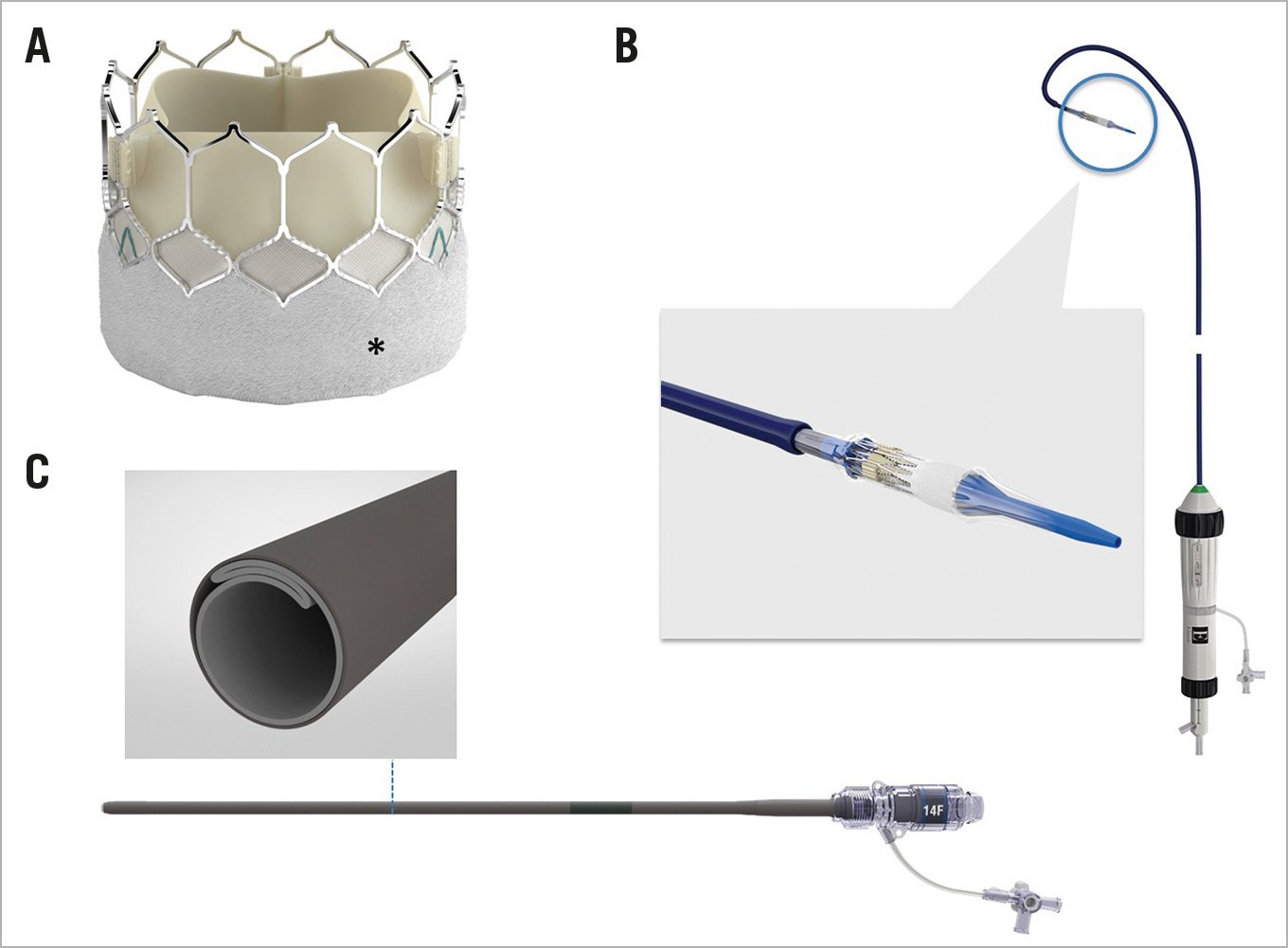
Figure 1. The SAPIEN 3 Ultra device. The SAPIEN 3 Ultra presents a number of important innovations in comparison with the SAPIEN 3 device, that involve the valve, the delivery system, and the introducer sheath. A) The valve retains the SAPIEN 3 frame and bovine pericardial leaflets, whereas the outer skirt (asterisk) is made of textured PET, different from the fabric seal of the SAPIEN 3 and around 40% higher. B) The Ultra delivery system. Highlighted, the new balloon re-designed to allow on-balloon valve crimping, obviating the need for valve alignment and pusher retraction before valve release. The distal end presents a smoother tip-to-valve transition and a shorter tapered distal tip. C) The Axela sheath: 14 Fr expandable sheath compatible with all valve sizes, engineered to allow transient expansion and active contraction (square box), with hydrophilic coating and a seamless design.
DEFINITIONS AND OUTCOMES
All patients had symptomatic severe native AS defined by standard criteria. Peripheral artery disease (PAD) included a history of intermittent claudication, previous peripheral vascular treatment or documented peripheral arterial stenosis greater than 50%. Chronic obstructive pulmonary disease was identified by forced expiratory volume in one second <1 litre or long-term use of bronchodilators, steroids or oxygen for lung disease. Chronic kidney disease (CKD) was identified by a glomerular filtration rate (GFR) <60 ml/min calculated by the Cockcroft-Gault formula. The logistic EuroSCORE (European System for Cardiac Operative Risk Evaluation), the EuroSCORE II and the Society of Thoracic Surgeons predicted risk of mortality (STS PROM) score were reported as part of a multiparametric evaluation12,13. TAVI procedures were performed as per the standard practice of each centre. There was no standardisation of post-procedural antithrombotic therapy. The primary objective of the study was to evaluate post-procedural and 30-day safety and efficacy outcomes. All endpoints were defined according to the Valve Academic Research Consortium-2 (VARC-2) criteria14. The main outcomes of interest were device success, all-cause death, cardiac death, stroke, vascular complications, bleeding, new permanent pacemaker insertion and acute kidney injury. Implant success was defined as only one valve implanted in the proper anatomical location, and device success according to VARC-2 criteria. All events and values collected are site-reported. The 30-day outcome was obtained through an outpatient clinic visit or telephone contact.
STATISTICAL ANALYSIS
Descriptive statistics are reported. Continuous variables were expressed as mean±standard deviation (SD), or median and interquartile range (IQR) when appropriate, and categorical variables as counts and percentages. All analyses were performed with the SAS 9.3 system (SAS Institute, Cary, NC, USA). Prospective TAVI databases were approved by the local ethics committee at each participating centre, and patients signed written informed consent for enrolment in the registry where required. The study protocol is in accordance with the Declaration of Helsinki. For the UK, data were collected as part of a mandatory UK national cardiac audit and all patient identifiable fields were removed before analysis. The study complies with section 251 of the National Health Service Act 2006. Ethical approval was not required under research governance arrangements for analyses.
Results
During the study period, 139 consecutive patients underwent transfemoral TAVI with the SAPIEN 3 Ultra at participating centres and were enrolled in the registry. Baseline characteristics are reported in Table 1 and Table 2. Mean age was 81.4±8.3 years, 54.5% were female and the average STS score was 3.8±2.4%. The vast majority of subjects (97.2%) underwent TAVI with local anaesthesia (28.8%) or by conscious sedation (68.3%) (Table 3). The new SAPIEN 3 Ultra valves 20, 23 or 26 mm were used in 118 patients (84.9%), and the SAPIEN 3 29 mm with the Axela sheath and the Ultra delivery system in 21 (15.1%). In one patient, the 23 mm valve got stuck in the sheath and could not be further advanced, requiring a sheath exchange and replacement of the valve with a new one. The procedure ended successfully but there was a flow-limiting dissection of the iliac artery that had to be fixed with a peripheral stent from the contralateral femoral artery. Re-analysis of the angio-CT scan confirmed a minimal diameter of 5.5 mm without relevant calcifications or tortuosity at the site of sheath kinking. Most of the valves were implanted without balloon predilatation (78.4%). Post-dilatation was performed in three cases (2.2%). Mean procedural duration, from entrance to exit from the catheterisation laboratory, was 121±47 minutes. Implant success was 100%, and device success according to the VARC-2 definition was 97.8%; there were two cases (1.4%) with moderate paravalvular leak (PVL), and one case (0.7%) with a mean final gradient >20 mmHg and patient-prosthesis mismatch (aortic valve area [AVA] <0.65 cm²/m²) with a 20 mm device. In-hospital, there were no cases of death, stroke, or conversion to open heart surgery. The rate of new permanent pacemaker implantation to discharge was 2.2%. Major vascular complications occurred in three patients (2.2%), and major or life-threatening bleedings in three patients (2.2%). Valve haemodynamics and incidence of PVL are shown in Figure 2. Mean transvalvular gradients were 19±6 mmHg for the 20 mm device, 12±4 mmHg for the 23 mm, 11±4 mmHg for the 26 mm, and 10±4 mmHg for the 29 mm. In one patient, a focal leaflet thickening suggestive of thrombosis was detected at post-procedural echocardiography, without affecting transvalvular gradients. The thickening resolved after 24 hours of full anticoagulation and the patient was discharged with a mean gradient of 10 mmHg. The median length of stay after TAVI was three days (IQR 3-5 days), and total hospitalisation was four days (IQR 2-7 days). At 30 days (Table 3, Figure 3), there were no deaths, MI, or strokes. Antithrombotics at discharge were dual antiplatelets in 58%, single antiplatelets in 7%, and oral anticoagulation in 35% (20% direct anticoagulants, in 10% combined with a single antiplatelet). Three additional patients required a new permanent pacemaker after discharge, with an overall 30-day incidence of 4.4%, and three patients were re-admitted because of congestive heart failure (CHF) (two paroxysmal atrial fibrillation [AF] with high heart rate, and one obese and severely hypertensive patient who inappropriately reduced diuretics). In all patients who were re-admitted, echocardiography showed a well-functioning prosthesis.
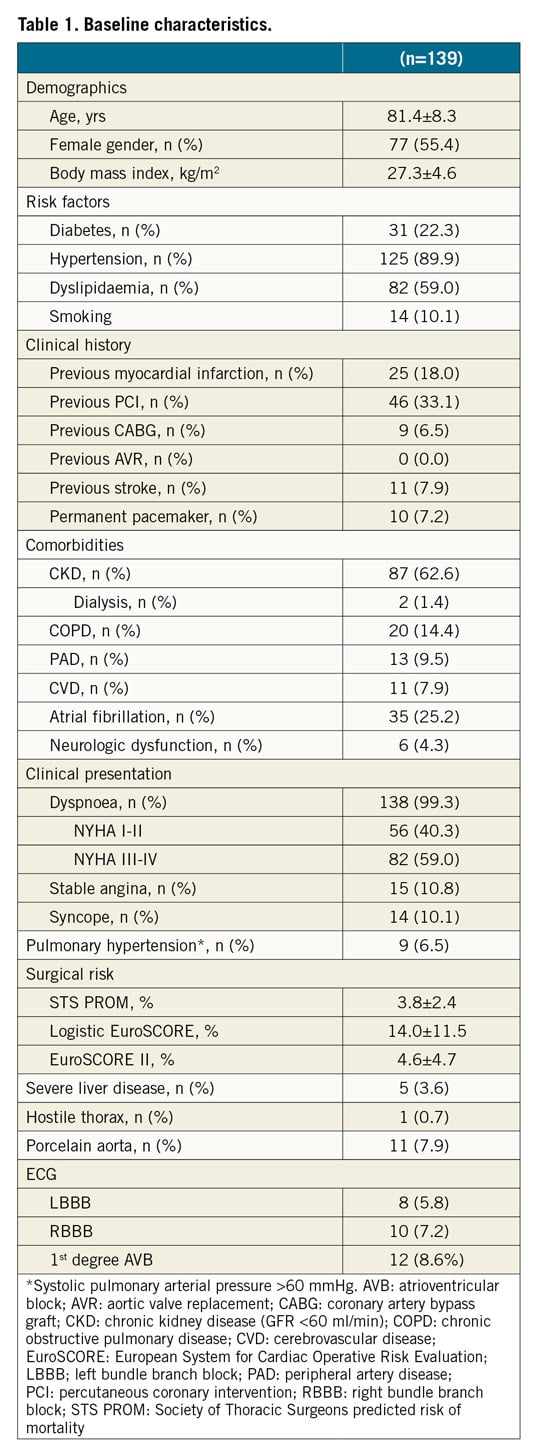
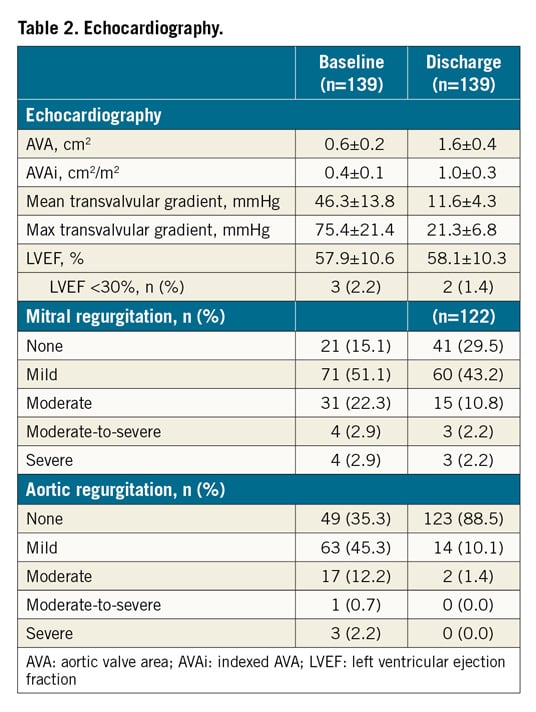
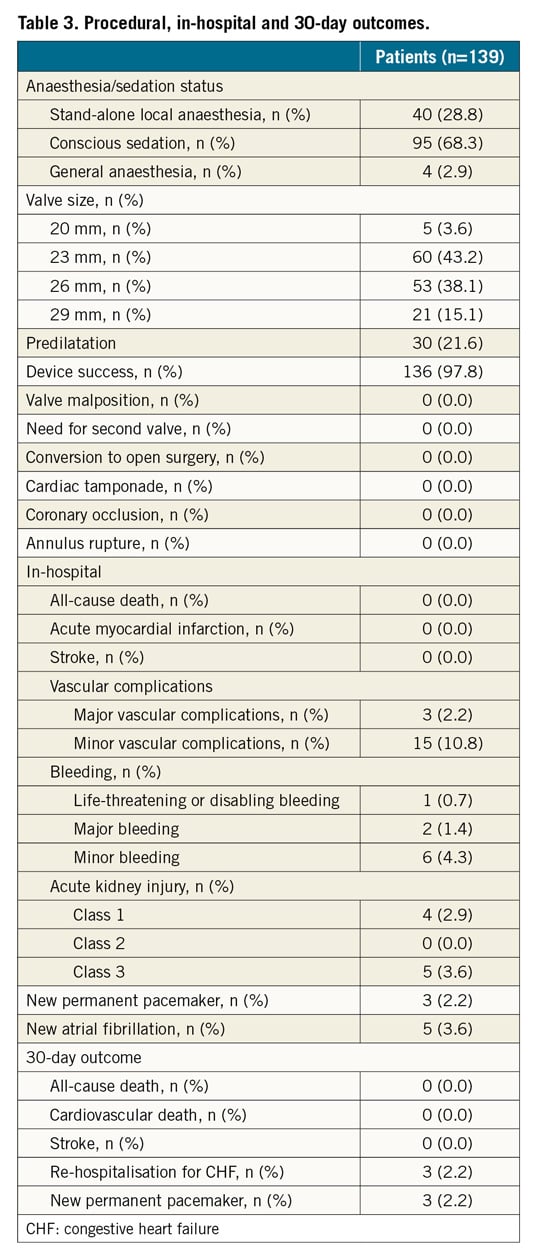
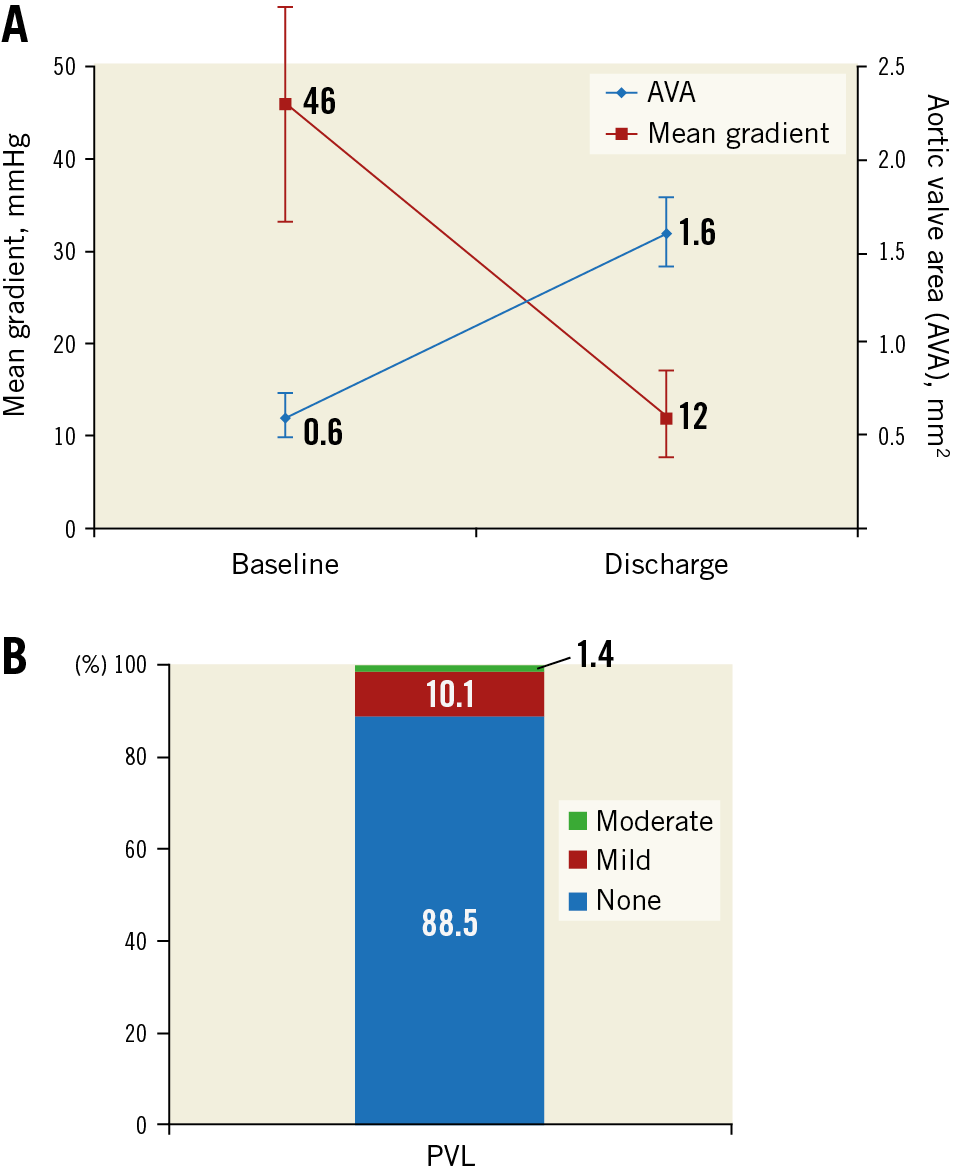
Figure 2. Haemodynamics and paravalvular regurgitation (PVL). A) Aortic valve area (AVA) before and after transcatheter aortic valve implantation at echocardiography. B) PVL grade before hospital discharge.
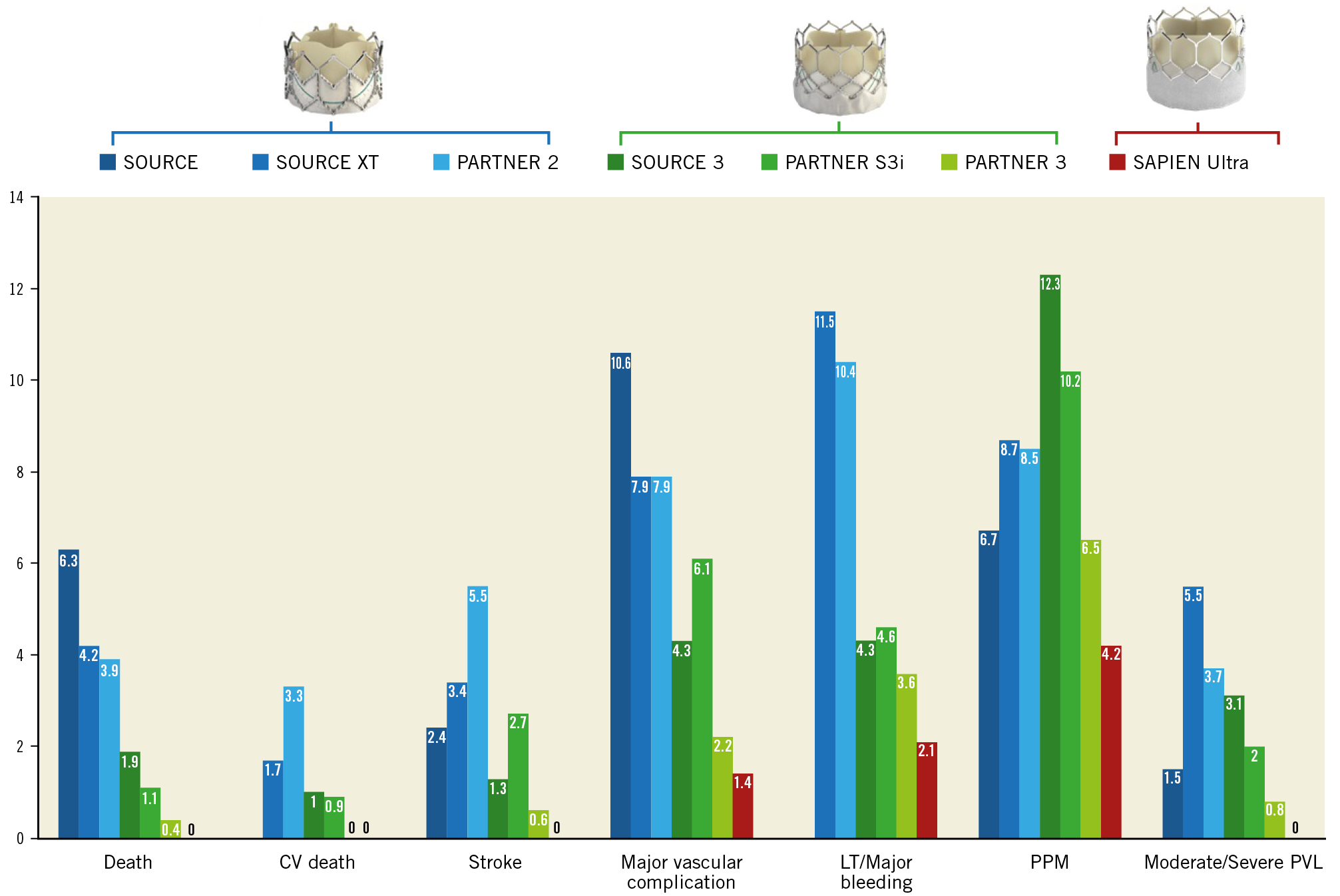
Figure 3. Thirty-day clinical outcomes. Thirty-day results of the SAPIEN 3 Ultra (S3U) registry in comparison with the main trials and registries of the SAPIEN transcatheter heart valve family.
The present patient population was compared with a control population composed of 139 consecutive patients treated with the SAPIEN 3 in the period immediately preceding the introduction of the Ultra system. The results are shown in Supplementary Table 1 and Supplementary Table 2. Briefly, patients treated with the Ultra were more frequently female with an overall lower surgical risk (e.g., STS PROM 3.8±2.4% vs 6.1±5.0%). There were no significant differences regarding in-hospital and 30-day outcomes.
Discussion
The current analysis reports for the first time on procedural and clinical outcomes in a consecutive series of patients undergoing transfemoral TAVI with the SAPIEN 3 Ultra in the prospective, international, multicentre S3U registry. The main findings are the following. 1) Device success was observed in 98% of the cases. 2) In-hospital, there were no cases of death, stroke or conversion to open heart surgery. The PPM rate, major vascular complications and severe bleeding were below 3%, and PVL below 2% (none >moderate). 3) Valve haemodynamics seem comparable to the SAPIEN 3. 4) These results were maintained at 30 days with the final incidence of new PPM being 4.4%. In addition, the rate of post-dilatation was low, compared with modern series, at 2.2%, perhaps indicating the better sealing characteristics of the improved PET skirt.
The technical changes of the SAPIEN 3 Ultra compared to the SAPIEN 3 were aimed at streamlining the procedure, making it safer, expanding the number of patients treatable using the transfemoral access and, most importantly, reducing the incidence of PVL. Our initial experience, with strict on-label use of the SAPIEN 3 Ultra, confirms these expectations in terms of both early procedural and clinical results. Of note, general anaesthesia was not needed in 97.2%, balloon predilatation was performed in a minority of patients, and the median hospital length of stay after TAVI was three days. Most importantly, we report an exceptionally low incidence of all major complications both in-hospital and at 30 days that parallels those reported by Waksman and colleagues with the use of the SAPIEN 3 in low-risk patients15. However, we enrolled older patients (on average +8 years) and, differently from the low-risk TAVR study, we included patients across all risk categories. There was, however, one case with a small kinking of the sheath that was not visible on angiography but precluded valve advancement, finally causing a minor vascular complication. Whether this could be attributed to the play of chance or to a somewhat minor resistance to compression of the Axela sheath cannot be inferred from our data. After completion of this study, other similar cases have been reported worldwide. In addition, a few cases of unexplained balloon rupture during valve implant have been reported which have resulted in significant difficulty retrieving the valve into the catheter and withdrawing the system from the patient. In some cases, this was associated with vascular injury, bleeding, or need for surgical intervention, prompting the manufacturer to issue a Field Safety Notice and, later on, the US Food and Drug Administration (FDA) to issue a class I recall of the SAPIEN 3 Ultra delivery system16. Following these events, the company is now supporting the use of the SAPIEN 3 Ultra with the Edwards Commander delivery system through the 14 Fr expandable eSheath.
In contrast with previous studies which have observed a higher rate of pacemaker requirement with the SAPIEN 34,11,17 compared to the SAPIEN XT trials2,10, the rate of PPM implantation in our study was low at 4.4% to 30 days. The reasons for this observation are speculative and may have occurred by chance or be explained by the small cohort size. Our patient population presented a low rate of right bundle branch block (RBBB) or conduction disturbances at baseline. However, it is possible that the low rate of predilatation, afforded by the lower crossing profile of the device and the low rate of post-dilatation, due to the improved sealing skirt reducing PVL, may have been contributory factors.
Limitations
This study presents some limitations that should be acknowledged. This is a relatively small series of SAPIEN 3 Ultra-treated patients performed by very experienced operators working in high-volume centres. Second, outcomes were self-reported by participating centres in the absence of a clinical events committee, and there was no core lab evaluation of echocardiographic results. While hard clinical endpoints such as mortality, stroke and major bleedings can be considered highly reliable, echocardiography and haemodynamics could suffer from inter-centre differences in evaluation and reporting. Overall, the external validity of these results should be evaluated in larger studies. Importantly, however, our results were obtained in the learning phase of the new device for all operators, confirming the safety and the efficacy of the SAPIEN 3 Ultra even under these circumstances. The comparison with previous-generation devices (Figure 3) is provided only for descriptive purposes and has no scientific validity. In fact, we cannot assume that the improved outcomes are due only to the device because the time period is different, and it is well established that outcomes are improving due to increased experience. Finally, in the present study, all cases were performed with the Axela sheath and the Ultra delivery system. After identification of potential issues with both devices, in most centres the SAPIEN 3 Ultra valve is now implanted using the SAPIEN 3 delivery kit consisting of the eSheath and the Commander delivery system. Although it is reasonable to assume consistent results with the latter combination, further testing is needed to confirm this hypothesis.
Conclusions
This first multicentre experience of patients treated by transfemoral TAVI with strict on-label use of the new SAPIEN 3 Ultra transcatheter heart valve shows good in-hospital and 30-day clinical outcomes, confirming that refinements in technology and biomedical engineering may simplify and improve overall TAVI results.
|
Impact on daily practice Transcatheter aortic valve implantation (TAVI) represents an alternative to surgical aortic valve replacement (SAVR) for elderly patients with severe aortic stenosis (AS) irrespective of surgical risk. TAVI devices are constantly being refined to address shortcomings of previous generations. This investigator-driven multicentre international study is the first to evaluate in-hospital and 30-day clinical outcomes associated with the latest-generation balloon-expandable transcatheter heart valve of the SAPIEN family, the SAPIEN 3 Ultra, demonstrating effective reduction of short-term complications and good clinical results. |
Conflict of interest statement
F. Saia reports receiving consulting fees from Abbott Vascular, Eli Lilly, AstraZeneca, St. Jude Medical, and speaker’s fees from Abbott Vascular, Eli Lilly, AstraZeneca, St. Jude Medical, Terumo, Biosensors, Edwards, and Boston Scientific, and personal fees from Edwards, outside the submitted work. G. Tarantini reports speaker’s fees from Abbott Vascular, Edwards, Boston Scientific, and Medtronic. S.N. Doshi has received consulting fees from Medtronic, Edwards Lifesciences and Boston Scientific, and is a proctor for Edwards Lifesciences. M. Laine reports consulting fees from Boston Scientific, Medtronic and Edwards Lifesciences. F.L. Ribichini is a proctor for Edwards and reports personal fees from Edwards, outside the submitted work. T. Palmerini reports personal fees from Edwards, outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

