Abstract
Building on the established success with the SAPIEN, SAPIEN XT and earlier prototypic transcatheter heart valves (THV) the newest balloon-expandable valve incorporates a number of new and enhanced features intended to reduce the risk of vascular injury, to reduce paravalvular regurgitation, and to facilitate rapid and accurate positioning and implantation. The SAPIEN 3 THV incorporates a cobalt chromium stent, bovine pericardial leaflets, and both an inner and new outer polyethylene terephthalate sealing cuff. The delivery system incorporates an active three-dimensional coaxial positioning catheter, and is compatible with a 14 Fr expandable sheath.
Introduction
The first-in-human transcatheter aortic valve implantation (TAVI) was performed with a balloon-expandable transcatheter heart valve (THV) ten years ago1. Today TAVI is generally accepted to be the intervention of choice for symptomatic severe aortic stenosis in “inoperable” patients2,3 and a reasonable alternative to surgical aortic valve replacement in selected “high-risk operable” patients4,5. The SAPIEN 3™ (S3) (Edwards Lifesciences, Irvine, CA, USA) is a new balloon-expandable THV, which incorporates features to reduce vascular complications, ensure paravalvular sealing, and enhance ease of positioning.
Evolution of balloon-expandable transcatheter heart valves
The first THV intended for permanent implantation6 was handmade of stainless steel surgical wires folded in loops with a porcine aortic valve sewn inside (Figure 1). Successful implantation in a porcine model was reported in 1992 establishing the feasibility of TAVI using a retrograde, arterial approach with a balloon-expandable, catheter-mounted THV. The THV implanted in the first-in-human TAVI experiences1,7 ten years later (Cribier-Edwards valve; Edwards Lifesciences) (Figure 2) was constructed from a stainless steel stent, equine pericardial leaflets and a fabric cuff that covered the internal bottom third of the prosthesis. For the first-in-human procedure1 the THV was directly crimped on a standard valvuloplasty balloon, although subsequently the deployment balloon was incorporated into a deflectable guiding catheter7. The successor of the Cribier-Edwards THV was the SAPIEN THV (approved for clinical use in the United States and Europe, Figure 2). The stent remained essentially unchanged, although the leaflets were now constructed of bovine pericardial tissue. The ThermaFix™ process was applied to the pericardial tissue to remove calcium binding sites in order to reduce calcium uptake and increase durability. Along with improvements in THV design, modifications were made to the delivery systems (Figure 3), the tip of the balloon catheter (nose cone), the system profile and controllability. While the SAPIEN XT THV (currently used in the PARTNER II trial and approved for clinical use in Europe, Figure 2) is also comprised of treated bovine pericardial leaflets, the stent material was changed to cobalt chromium. With changes in design and materials the THV profiles could be downsized, which reduced the risk of vascular complications. While the 23 mm and 26 mm Cribier-Edwards and SAPIEN THVs required 22 Fr and 24 Fr sheaths, respectively, the SAPIEN XT 23 mm and 26 mm are accommodated by 18 Fr and 19 Fr sheaths, respectively. The stress on the access vessel could be further reduced with the introduction of expandable sheaths. Currently the SAPIEN XT 23 mm, 26 mm and 29 mm can be delivered by 16 Fr, 18 Fr and 20 Fr expandable sheaths, respectively.
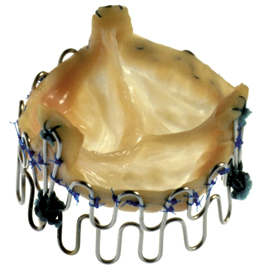
Figure 1. Pioneering assembly of the first balloon-expandable transcatheter heart valve in 1992. The stent of this handmade transcatheter heart valve (THV) was constructed with two 0.55 mm stainless steel wires folded in 15 loops. A three-leaflet porcine aortic valve (from the local butcher) was sutured inside the stent. The THV was crimped on a three-foiled balloon dilation catheter (external diameter of the collapsed THV 12 mm) and was expanded by balloon inflation in a porcine model to 32 mm. (Reprinted with permission from Dr Henning Andersen)
The SAPIEN 3
The S3 THV (Figure 2 and Figure 4) incorporates a unique stent and leaflet design that allows for crimping to a further reduced profile as compared to the Cribier-Edwards, SAPIEN and SAPIEN XT THVs. As with its predecessors, the inflow of the S3 is covered by an internal polyethylene terephthalate (PET) skirt. However, this new THV incorporates an additional outer PET sealing cuff intended to reduce paravalvular regurgitation. For the first-in-human experience the S3 THV was available in a 26 mm version (height of 20 mm when fully expanded), but additional sizes (20 mm, 23 mm and 29 mm) are anticipated.
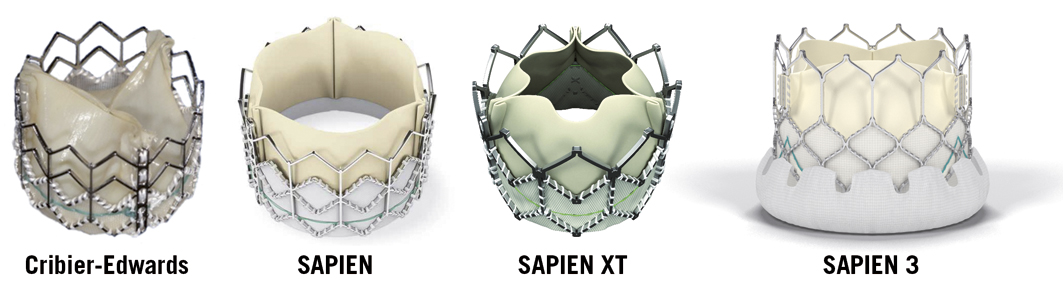
Figure 2. Evolution of balloon-expandable transcatheter heart valves. The Cribier-Edwards valve consists of a stainless steel stent and equine pericardial leaflets and was implanted in the first-in-human TAVI experiences. The SAPIEN is an approved transcatheter heart valve comprising a stainless steel frame and bovine pericardial leaflets (sizes 23 mm and 26 mm). The successor (SAPIEN XT, manufactured sizes 20 mm, 23 mm, 26 mm and 29 mm) is currently evaluated in the PARTNER II trial and approved for clinical use in Europe. It is constructed of a cobalt chromium stent, which allows crimping to a lower profile. The SAPIEN 3, which also comprises a cobalt chromium frame and trileaflet bovine pericardial tissue, has a further reduced profile and the inflow of the valve is covered by an outer polyethylene terephthalate sealing cuff, intended to improve paravalvular sealing. (Courtesy of Edwards Lifesciences, Irvine, CA, USA)
The delivery system
The delivery system (Commander; Edwards Lifesciences, Irvine, CA, USA) (Figure 3 and Figure 5) is a further development of the NovaFlex delivery catheter (Edwards Lifesciences), which is currently used for TAVI with the SAPIEN XT. The specially designed nose-cone-tipped inner balloon catheter on which the prosthesis is crimped has radiopaque valve alignment markers defining the valve position and the working length of the balloon. A central radiopaque marker aids valve positioning. The outer deflectable flex catheter is attached to the handle, which incorporates a wheel to deflect the flex catheter tip, an indicator which indicates the degree of tip flexion, a wheel (black) for fine alignment of the THV during valve positioning and a balloon lock knob to secure the balloon catheter to the flex catheter. Rotating the fine alignment wheel allows for advancing or retracting the valve several millimetres up or down within the annulus without pushing or pulling on the delivery system (Figure 5).
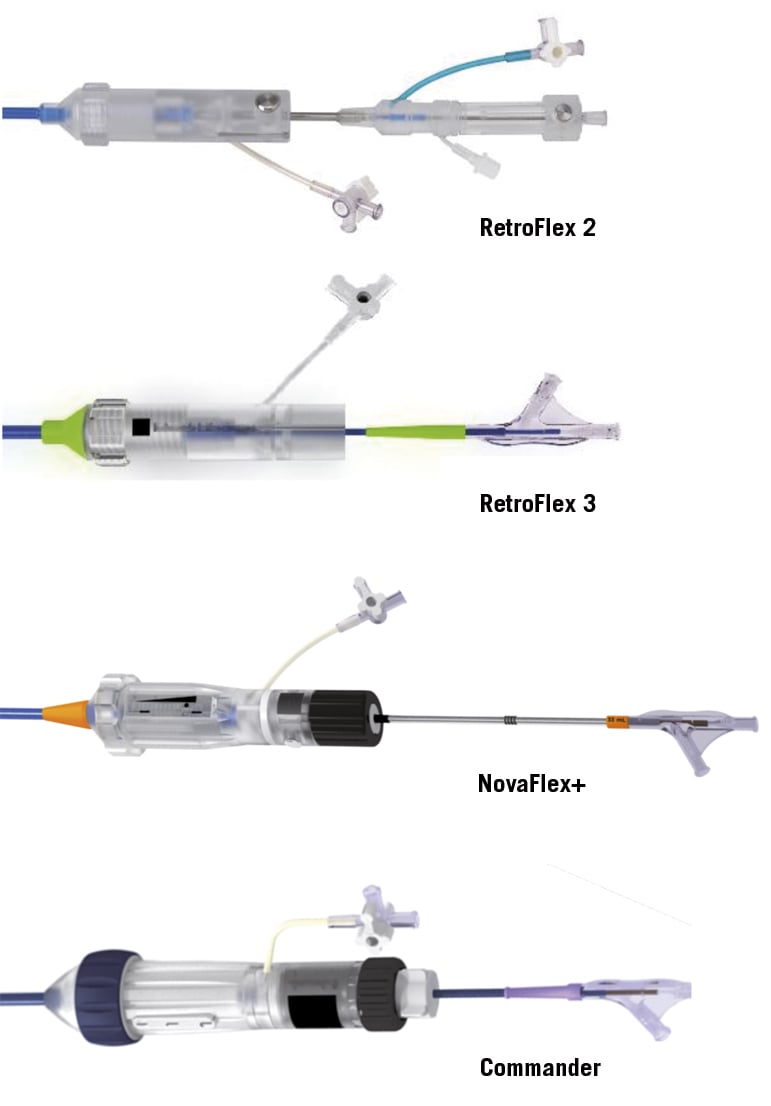
Figure 3. Evolution of delivery systems. After the initial experience with the RetroFlex 1 (not shown) and the Cribier-Edwards valve (Figure 2), a nose cone at the catheter tip to facilitate crossing of the native valve was introduced for the Retroflex 2. The length of the nose cone was reduced, to avoid left ventricular injury, for the RetroFlex 3, which is currently in clinical use for implanting the SAPIEN transcatheter heart valve (THV). A substantial reduction of the delivery system profile could be achieved by crimping the SAPIEN XT THV proximal to the balloon on the catheter and aligning the THV on the balloon inside the descending aorta (NovaFlex+). A new feature of the delivery system for the SAPIEN 3 THV (Commander) is the possibility to fine align the THV in the aortic annulus by rotating the fine alignment wheel (black wheel).
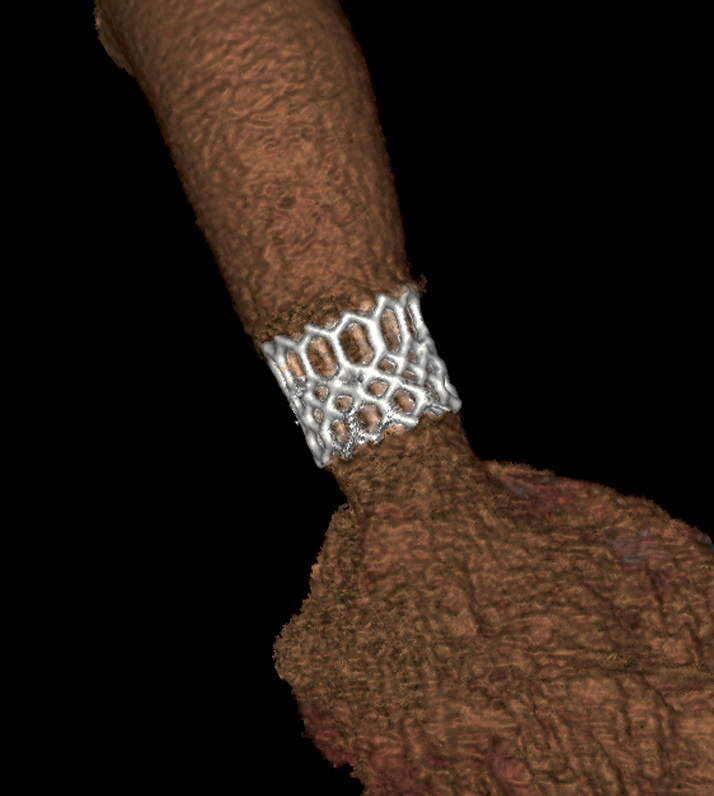
Figure 4. Three-dimensional reconstruction of the SAPIEN 3. The post-implant computed tomography sagittal oblique maximum intensity projection shows the SAPIEN 3 transcatheter heart valve seated in the native aortic valve.
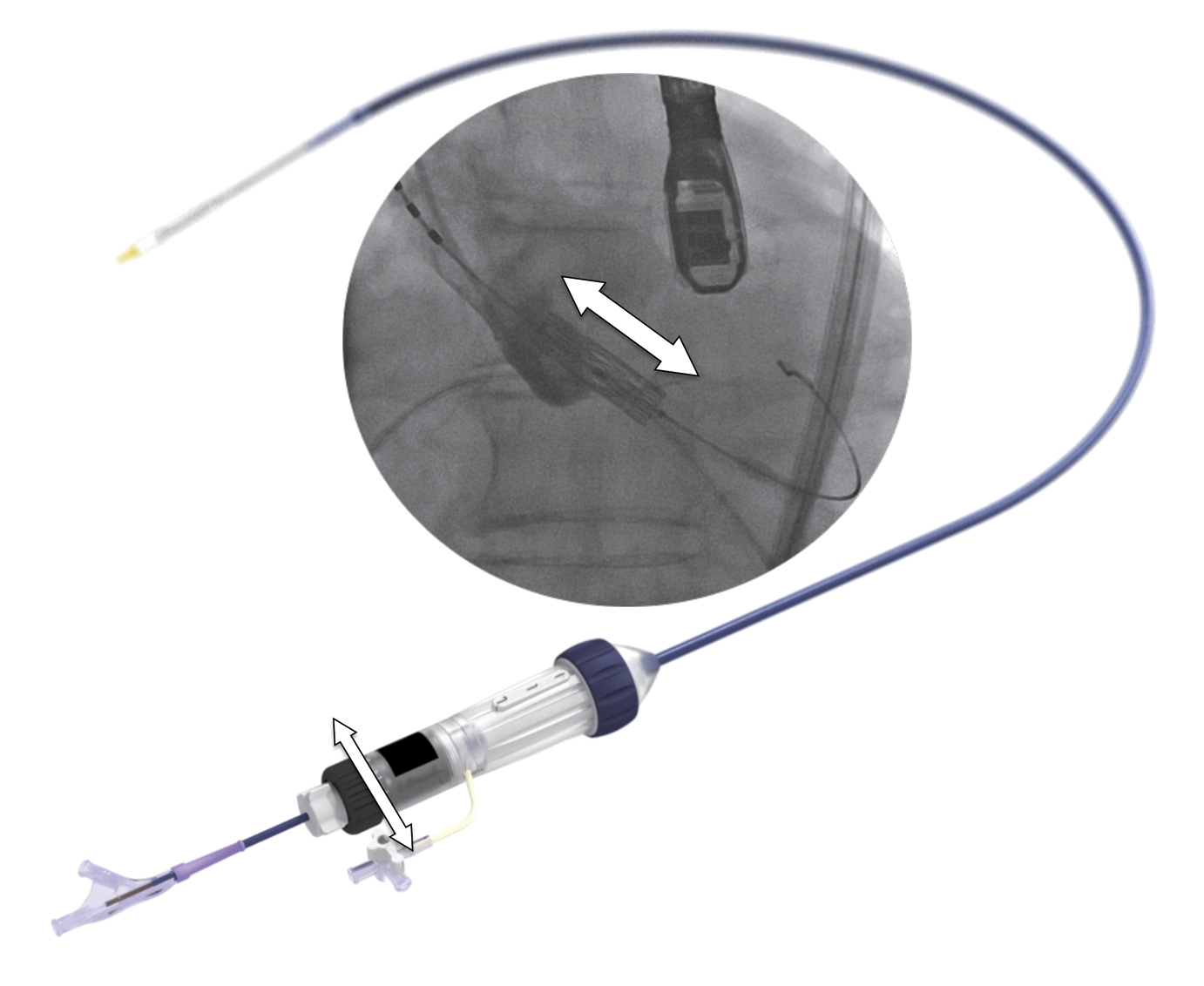
Figure 5. Features of the delivery system. The handle of the delivery system contains a wheel (black wheel with white arrows) for fine adjustment of the transcatheter heart valve during alignment. By rotating the fine adjustment wheel, the position of the crimped transcatheter valve can be precisely changed millimetre by millimetre, more aortic or ventricular in the aortic annulus, without pushing or pulling on the delivery system. The profile of the Commander (delivery system for the SAPIEN 3) is lower and its flexibility higher than the NovaFlex+ delivery system used for the SAPIEN XT.
The expandable sheath
The 26 mm S3 THV is compatible with a 14 Fr expandable sheath (eSheath; Edwards Lifesciences) (Figure 6). The expandable sheath reduces the stress on the access vessel by transiently expanding as the crimped THV passes through the sheath and then recoiling to a lower profile. This may reduce the potential for arterial injury during introduction.

Figure 6. The expandable sheath. The sheath temporarily expands to allow entry of the device, and subsequently recoils to a reduced profile as the SAPIEN 3 passes. This minimises the force required to insert and pass the delivery system and THV through the sheath, while maintaining a reduced profile in comparison to standard, non-expandable introducer sheaths.
Clinical implications
The S3 is a low-profile THV with features to reduce vascular complications and paravalvular regurgitation, while enhancing ease of positioning.
Vascular complications are a major cause of morbidity and mortality after TAVI8. In the PARTNER IB trial, major vascular complications were observed in 16.2% of patients undergoing TAVI with the SAPIEN THV3. The strongest predictor for vascular complications is the ratio of the outer diameter of the sheath to the minimal lumen diameter of the access vessel8. Downsizing THV delivery systems reduces the risk of vascular complications and translates into better TAVI outcomes. While the 26 mm SAPIEN requires a 24 Fr sheath and the 26 mm SAPIEN XT a 19 Fr sheath (or 18 Fr expandable sheath), the 26 mm S3 is accommodated by a 14 Fr expandable sheath. Some patients previously considered unsuitable for femoral access because of small vessel diameters may safely undergo TAVI with the S3 in future.
Paravalvular regurgitation (PAR) after TAVI has been associated with increased morbidity and mortality9. Moderate or severe PAR was reported in 12.2% of TAVI patients in the PARTNER IA cohort5 and in 13.9% in the FRANCE 2 registry10. Recently, an association of mortality with mild PAR was proposed4, but a cause and effect relationship was not established. One of the innovations of the S3 is the outer PET sealing cuff, which is intended to ensure paravalvular sealing. Reduction in PAR accomplished by this paravalvular sealing feature, but also by more accurate positioning, may lead to improved clinical outcomes.
Atrioventricular block necessitating permanent pacemaker implantation is a known potential complication of TAVI. The UK TAVI11 and FRANCE 210 registries reported new pacemaker rates of 7.4% and 11.5% with the SAPIEN/XT valves, respectively. These rates are significantly lower than after implantation of the self-expanding CoreValve (Medtronic, Inc., Minneapolis, MN, USA)10,12, which creates more stress on the atrioventricular conduction system. It is unknown whether the increased length of the S3 and the potential extension of the area of contact with the septum will increase the risk of atrioventricular block. In contrast, more accurate positioning and a lesser need for oversizing due to the paravalvular sealing cuff may reduce rates of permanent pacemaker implantation after TAVI with the S3.
Accurate positioning of THVs ensures adequate anchoring and sealing within the aortic annulus. An increased THV length facilitates optimal positioning within the native aortic valve. The expanded length of the 26 mm SAPIEN is 16 mm, of the 26 mm SAPIEN XT it is 17 mm, and of the 26 mm S3 it is 20 mm. In addition to this broadened landing zone of the S3, the delivery system of the S3 has several improvements to facilitate accurate positioning. The flexion capabilities were improved compared to the earlier NovaFlex system: this facilitates engaging the native valve in a coaxial manner. In addition, with the fine alignment mechanism, the height of the implant can be adjusted precisely (Figure 5).
Conclusion
The low profile of the SAPIEN 3 transcatheter heart valve will allow fully percutaneous implantation in a broader range of patients, and its features are anticipated to ensure more accurate positioning and less paravalvular regurgitation.
Conflict of interest statement
R.K. Binder, J. Rodés-Cabau, J.G. Webb and D.A. Wood are consultants to Edwards Lifesciences.

