
Introduction
Percutaneous pulmonary valve implantation (PPVI) with SAPIEN valves offers promising results, but it remains technically challenging1,2. The valve is introduced into a short sheath and advanced uncovered to the right ventricular outflow tract (RVOT). Tricuspid valve injury has been reported during this manoeuvre3. We describe a protective delivery method of the SAPIEN 3 valve (S3; Edwards Lifesciences, Irvine, CA, USA) in the pulmonary position.
Methods
Sixteen consecutive patients underwent PPVI of S3 valves using this modified procedure. The S3 is implanted using a Commander® delivery system (Edwards Lifesciences). It requires a 14 or 16 Fr short expandable sheath. During valve insertion, the sheath expands to an outer diameter of 23-27 Fr. We modified the procedure by using a 65 cm, 26 Fr, GORE® DrySeal Flex Introducer Sheath (W.L. Gore & Associates, Inc., Flagstaff, AZ, USA).
After the risk of coronary artery compression was excluded, the 26 Fr sheath was inserted percutaneously from the femoral vein to the distal pulmonary artery trunk. An AndraStent® XXL (Andramed GmbH, Reutlingen, Germany) or LDmax stent (ev3 Inc., Plymouth, MN, USA) was manually crimped onto a BIB® balloon catheter (NuMED Canada, Inc. Cornwall, ON, Canada), advanced through the protective 26 Fr sheath and implanted (Figure 1). The sheath was then carefully advanced inside the stent. The S3 was manually crimped onto the Commander system, oriented for antegrade deployment. The valve was inserted into the DrySeal sheath (Moving image 1) and advanced to the landing zone. The sheath and pusher of the delivery system were withdrawn, and the valve deployed (Figure 2, Moving image 2). At the end of the procedure, a subcutaneous purse-string stitch was placed at the venous access site.
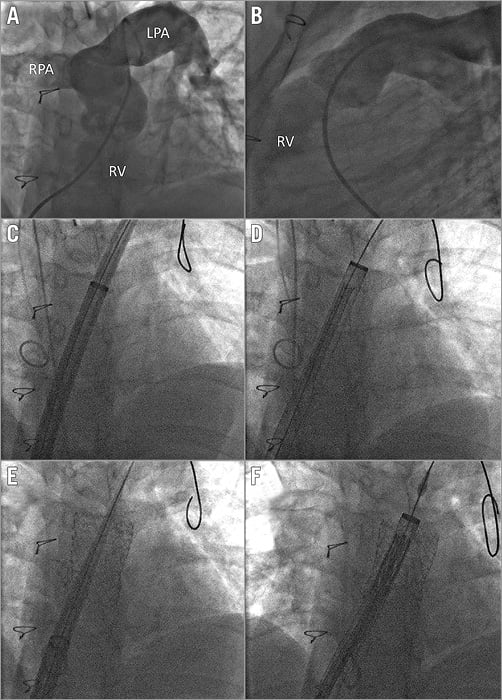
Figure 1. RVOT pre-stenting. Left anterior oblique (A) and lateral (B) views of the landing zone. C) A 65 cm, 26 Fr sheath is advanced with its introducer. D) An AndraStent is advanced. E) The 26 Fr sheath is moved forward with its introducer inside the stent. F) An S3 valve is advanced. LPA: left pulmonary artery; RPA: right pulmonary artery; RV: right ventricle
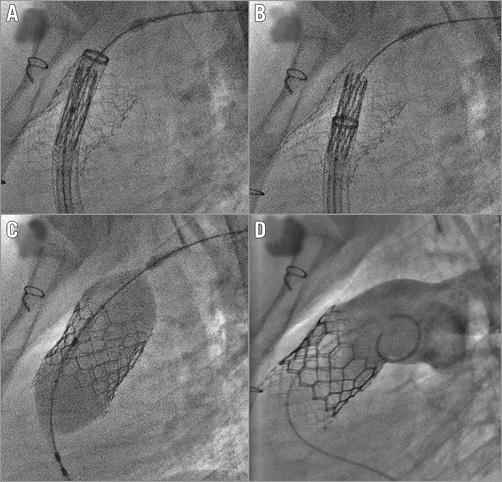
Figure 2. Valve implantation. A) An S3 valve is moved towards the stent protected by a 26 Fr sheath. B) Valve uncovering. C) Valve deployment. D) Final pulmonary angiogram.
Results
BENCH TESTING
A 29 mm S3 valve mounted on a Commander system was able to be inserted and reintroduced inside the 26 Fr sheath. These manoeuvres were not feasible with a 29 mm SAPIEN XT valve (Edwards Lifesciences).
PROCEDURES
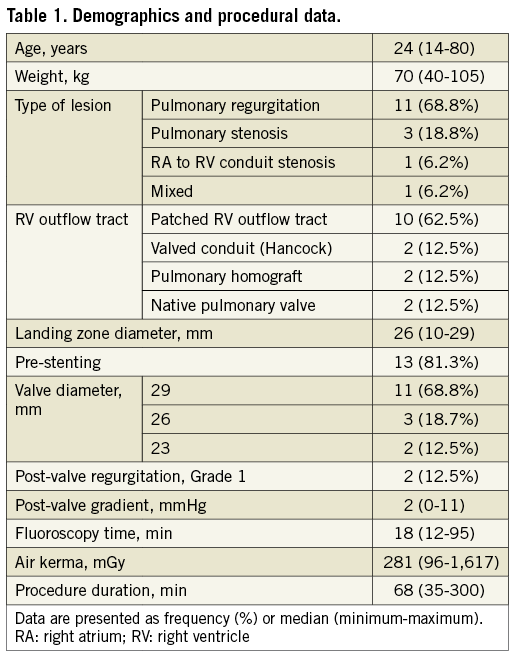
The procedural characteristics are reported in Table 1. A jugular vein approach was required in one case to advance the 26 Fr sheath in the pulmonary artery. In another patient who was 192 centimetres tall, the 26 Fr GORE® DrySeal sheath was not long enough to reach the end of the pulmonary trunk. The sheath was positioned as distally as possible just below the landing zone in the RVOT. Pre-stenting was carried out using an 80 cm, 18 Fr Check-Flo® sheath (Cook Medical, Bloomington, IN, USA) inserted inside the 26 Fr sheath. Valve implantation was successful.
Stent migration occurred in one case during stent crossing by the 26 Fr sheath. The stent was pulled back and further expanded in a good position. A small malformation of the stent during crossing with the sheath occurred in two cases but did not preclude valve implantation.
In the first case, the angulation of the RVOT prevented advancing the valve on the delivery balloon with the pusher. We removed the valve and the Commander system. A new valve was crimped directly onto the balloon of a new Commander delivery system and successfully deployed. In all subsequent cases, we positioned the valve directly onto the balloon.
Median post valve gradient was 2 mmHg. Trivial paravalvular leak was reported in two cases. Median procedure duration was 68 minutes (60-80) and median fluoroscopy time was 18 minutes (16-28). Vessel access bleeding was reported in four cases and resolved after prolonged compression without blood transfusion. Pre-discharge venous Doppler ultrasound was normal in all cases. Median post-intervention valve gradient was 2 mmHg. There were two trace to mild paravalvular leaks in two cases. No late complication was reported.
Discussion
We report a modified procedure using a 65 cm, 26 Fr delivery sheath allowing successful PPVI of S3 valves in 16 consecutive patients. The Melody valve and the Edwards SAPIEN XT valve have received CE mark and FDA approval for PPVI. The Ensemble® delivery system (Medtronic, Minneapolis, MN, USA) has proven to be a safe and a user-friendly delivery system for the Melody® valve (Medtronic), with a lower profile (outer diameter 22 Fr)4. The valve is covered by the delivery system and advanced safely across the tricuspid valve and the RVOT. Implantation of the Melody valve in a conduit larger than 24 mm is challenging. SAPIEN valve diameters, up to 29 mm, have expanded the feasibility of PPVI in larger conduits with good outcome1,2. The limited flexibility in the delivery system makes it more challenging for use in PPVI, given the circuitous course to the RVOT. Moreover, the valve cannot be easily removed in case of implantation failure. Tricuspid regurgitation has been reported as a complication of Edwards SAPIEN XT PPVI in three patients out of 40, with two requiring tricuspid surgery3. The use of a long, large-bore sheath prevents the introduction of the valve stent causing severe complications, including tear of the tricuspid valve.
Advancing the sheath into the stent after pre-stenting and before valve deployment was the most challenging procedural step. Alternatively, valve implantation could have been postponed until a second procedure to allow in-growth and stability of the stent.
Limitations
Larger studies are warranted to confirm our data. A limitation of this series is that patients weighed more than 40 kg in our study and the large-profile sheath may not be useful in smaller patients. The Melody valve could alternatively be used in these cases if the conduit diameter does not exceed 22 mm5. Vessel access haematomas were reported in four cases. Venous preclosure could have been considered.
Conclusion
Percutaneous pulmonary valve implantation of the S3 valve using a large delivery sheath is efficient and safe. Our study confirms the proof of principle of valve protection during PPVI.
| Impact on daily practice A long and large delivery sheath can be used during PPVI of the S3 valve to protect the tricuspid valve and to facilitate access to the landing zone. |
Conflict of interest statement
S. Haulon reports personal fees from Cook Medical and GE Healthcare, outside the submitted work. J. Petit reports personal fees from Edwards Lifesciences, outside the submitted work. S. Hascoet reports personal fees from Abbott, outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. An S3 on a Commander system is introduced into a 26 Fr sheath.
Moving image 2. Step-by-step modified procedure for PPVI of the S3 valve.

