Abstract
Percutaneous pulmonary valve implantation has been widely accepted as an alternative to surgery in selected patients with right ventricular outflow tract (RVOT) dysfunction. This totally new field of our specialty pushed centres to rethink overall strategies on how to treat RVOT dysfunction. In this review, we will focus on challenges related to patient selection, and discuss innovative procedural techniques developed over the years to enlarge the number of candidates for the technique.
Introduction
Percutaneous pulmonary valve implantation (PPVI) has been widely accepted as a less invasive and suitable alternative to surgery in selected patients. Since the first report in human from the Necker Hospital, Paris, France in 2000, PPVI has created a paradigm shift in cardiology1,2. A completely new field opened up, not limited to the pulmonary valve in congenital cardiology but largely extending to all segments of cardiology. The number of patients undergoing PPVI has been increasing over the years with the opening of new implanting centres throughout the world. There have been more than 10,000 pulmonary implants in more than two hundred centres over six continents. This new field of our specialty has led centres to reconsider interventional and surgical strategies on how to approach right ventricular outflow tract (RVOT) dysfunction. In this review, we will discuss challenges related to patient selection, and procedural techniques developed over the years.
Available valves
There are currently two different types of balloon-expandable valve CE marked for use in human. These are the Melody® (Medtronic, Minneapolis, MN, USA) and the older-generation Edwards SAPIEN XT (Edwards Lifesciences, Irvine, CA, USA) valves (Figure 1). The Melody valve gained CE mark in 2006 and represents the vast majority of valves implanted throughout the world. It is made of an original bovine jugular vein containing a valve. This natural valve functions in low-pressure conditions, at various diameters and geometries, i.e., the same valve of 18 mm diameter works perfectly well in a range of diameters from 14 mm to 24 mm as well as in round and oval shapes. Selected trileaflet valved segments are sutured to the frame of a balloon-expandable stent (CP 8Z34; NuMED, Inc., Hopkinton, NY, USA). There are two valve sizes, TPV20 and TPV22, that can be delivered using a 22 Fr dedicated delivery system (Ensemble®; Medtronic) which is available in three sizes (18, 20 and 22 mm balloon catheter). The outer diameter of the Melody when expanded to 22 mm is 24 mm. This valve can be expanded using a 24 mm balloon (giving an external diameter of 26 mm) without significant leakage3.
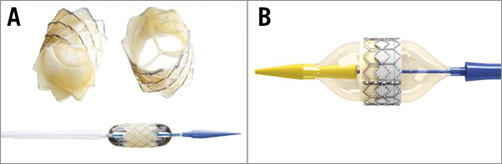
Figure 1. In vitro picture of two available balloon-expandable valves. A) Melody. B) SAPIEN.
The Edwards valve (SAPIEN XT) was originally developed for the aortic position. It is a manufactured valve made of bovine pericardium mounted in a cobalt-chromium stent shorter than the Melody (14.3 to 19.1 mm). Its introduction in the congenital field is more recent. CE mark was gained in 2016. It comes in diameters of 23, 26 and 29 mm (external diameters). Being artificial and made of three symmetric leaflets, it tolerates only minor eccentricities in size and geometry. The valve is delivered using a dedicated delivery system (NovaFlex; Edwards Lifesciences).
Indications and patient selection
Selecting patients is one of the crucial steps in this procedure. Lessons learned from right ventricular physiology and the damage caused by waiting and delaying repeated surgeries have improved our understanding on optimal timing for pulmonary valve replacement4-6. PPVI is now offered in a variety of congenital heart disease surgeries, which involves RVOT being repaired either directly with patch or indirectly with interposition of conduits or homografts. Two categories of patient are potential candidates, those with pulmonary stenosis with or without pulmonary regurgitation (PR), and those with pure PR. Most guidelines regarding timing for pulmonary valve replacement in pure PR rely on combined parameters including clinical symptoms, magnetic resonance imaging (MRI) data, decreased exercise capacity, QRS enlargement and arrhythmia. At the other end of the spectrum, relief of stenosis is considered in symptomatic patients with right ventricular (RV) systolic pressure >60 mmHg, or in asymptomatic patients with severe RVOT obstruction and one of the following criteria: RV systolic pressure >80 mmHg, progressive RV systolic dysfunction, or decrease in exercise capacity7. When criteria are met, it is important to define whether a patient is a candidate for PPVI or whether the better option is surgery. In that perspective, preprocedural assessment (transthoracic echocardiography along with MRI or CT scan) that helps to indicate pulmonary valve replacement is also very important to define what techniques should be used. MRI or sometimes CT scan permits an understanding of the anatomy and provides very important details on suitability, in particular in patients with patched RVOT. Besides RVOT dysfunction, the presence of multiple lesions (such as branch pulmonary artery stenosis, residual ventricular septal defect [VSD], tricuspid regurgitation [TR], etc.…) should be carefully assessed to verify whether they can be dealt with percutaneously. The presence of a TR is of particular interest because it usually accompanies RV dilatation. In our opinion, in the presence of TR greater than grade 3, surgery should be favoured because reduction of RV dilatation following PPVI is unlikely to correct such a degree of TR8. Special attention should be given to the coronary arteries and their relationship with the expected landing zone in the RVOT. However, no patient with close proximity should be contraindicated based on imaging. Because the direction of the RVOT opening cannot be anticipated, the final decision should be made in the cardiac laboratory.
Besides imaging, the most important consideration is clinical history. All prior operative notes should be reviewed to understand what the RVOT is made of. The information on type and size of the valve, if present, should be extracted and analysed with regard to the mode of degeneration. In obstructive lesions with or without PR, the question is not the feasibility of PPVI but the possibility of opening the RVOT with the goal of minimising RVOT gradient. Therefore, initial diameter and, most importantly, the nature (i.e., elasticity) of the surgical valve are very important to know and understand, as it has been demonstrated that patients with non-expandable conduits <20 mm in diameter showed poor midterm haemodynamic outcomes9. Patients with expandable conduits such as Contegra® (Medtronic) or homograft can benefit from PPVI as those substrates can be expanded from 12 mm to 22 mm (Figure 2)10. In a purely regurgitant lesion, the possibility of anchoring the percutaneous valve is the main question. Here again, the nature of the RVOT should be considered in terms of sizing and compliance. When a surgical valve is present, one should be aware that the labelled diameter of valves refers to the inner or outer diameter depending on the company. In patched RVOT, the dimensions of the RVOT may vary a lot between systole and diastole. Measurements with imaging should be made during both cardiac cycles, and a mean value should not be used as the target diameter for the valve to be implanted. In this setting, it is also important to analyse both pulmonary arteries as they can be used to anchor the valved stent better.
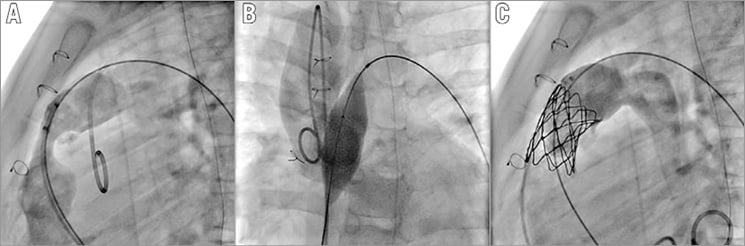
Figure 2. Patient with stenotic 12 mm Contegra conduit. A) Angiogram in the lateral view showing a tight stenosis and free PR of the conduit. B) Balloon interrogation with Atlas® 20 mm (Bard Peripheral Vascular, Tempe, AZ, USA) showing complete opening without coronary compression. C) Final angiogram showing good valve function.
Technical considerations
The technique has been extensively reported in multiple publications11-13. We will focus on specific considerations.
RVOT BALLOONING: SIZING OF THE RVOT AND CORONARY ARTERY EVALUATION
Balloon sizing is one of the major steps in the procedure. It allows estimating diameter, dilatability of the RVOT, as well as proximity of the coronary arteries. In patched regurgitant RVOT, balloon sizing is crucial to estimate RVOT diameter, select the proper valve and avoid embolisation of the device. A compliant balloon is generally used in that situation. We recommend inflating the balloon until an indentation is obtained concomitant to a drop of aortic pressure, then confirming by dye injection that no flow crosses the RVOT14.
Coronary compression is one of the major complications reported during PPVI with a potentially catastrophic outcome15,16. Patients at risk should be identified prior to proceeding to stent implantation. Correct balloon sizing is crucial for adequate interpretation. Otherwise, the operators will be falsely reassured. In stenotic lesions, a non-compliant balloon should be used and opened to the target diameter of the valve to be implanted (external diameter). An underexpanded balloon or the use of a smaller balloon does not exclude a potential coronary compression. The space between the RVOT and the coronary arteries is not empty, and tissue can be moved during balloon dilatation towards the coronary arteries and compress them even if these are initially distant from the RVOT. Any technique such as rotational angiography without adequate balloon testing, just giving a sense of position of the coronary arteries, will not be sufficient to evaluate the coronaries adequately. Any coronary compression should be analysed with regard to the expected landing zone, as the balloons used are generally long and stiff enough to modify the RVOT geometry. Our policy is to perform a baseline aortogram in two projections with the balloon across the RVOT inflated at “indentation” diameter. The aim here is to optimise the aortogram by reducing the cardiac output and to delineate the full coronary anatomy. A new aortogram in the same projections is then performed at full inflation of the balloon (no indentation should be seen) and compared with the previous one to look for compression or missing coronaries (Moving image 1). Occasionally, if there are still some concerns about proximity, other projections or selective angiograms must be considered. If, after careful evaluation, there are still doubts about coronary evaluation, two options can be considered. First, these patients should be sent to surgery, thus occulting the risk of coronary compression. The other option would be to be fully prepared to treat a coronary compression with a wire in place in the coronary artery, an adequate guiding catheter at the mouth of the coronary artery and a proper coronary stent ready for insertion on the table (Figure 3, Figure 4). The same choice applies to patients who previously underwent RVOT stenting. These patients cannot be assessed properly with balloon sizing, as any balloon dilatation would lead to irreversible expansion of the stent in place. Except for patients with definitive contraindication to surgery, we do not think that there is a place for coronary stenting in prevention of coronary compression with available devices.
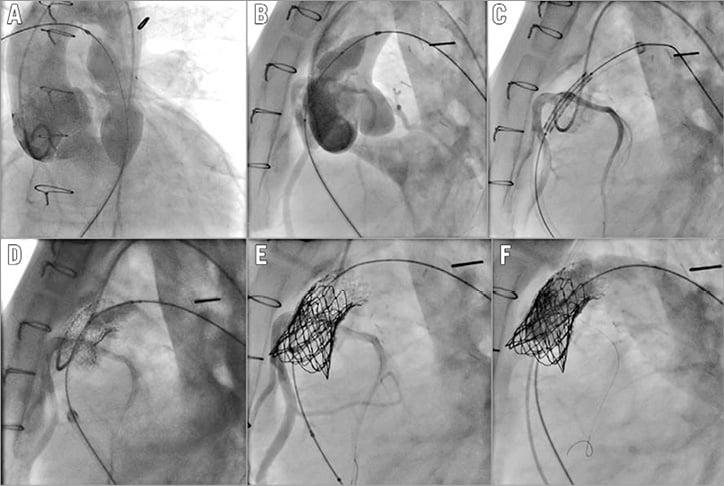
Figure 3. Patient with calcified homograft. The coronary arteries are proximal to the RVOT (A & B). A guidewire has been placed in the coronary artery for faster access to the vessel in case of compression during the procedure (C, D, & E). Coronary angiograms during BMS insertion showing patency of the vessel (D & E). F) Final angiogram showing perfect function of the Melody.
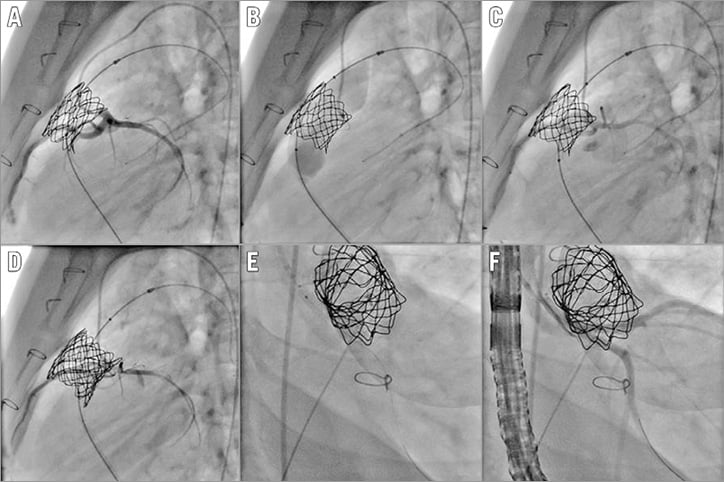
Figure 4. Patient with stent previously placed in the RVOT. Note the proximity of the left main coronary artery (A). Expansion of the stent with a wire placed in the LAD (B). C) Coronary angiogram after full opening of the stent showing patency of the vessel. D) Coronary angiogram after implantation of the Melody valve showing severe compression of the left coronary artery. E) Coronary stent being implanted. F) Final picture showing reopening of the coronary artery.
RVOT RUPTURE
The counterpart of adequate coronary testing is the risk of conduit rupture that has been reported to be as high as 9% of patients17. Most of these RVOT injuries are contained by surrounding scar tissue, with less than 2% having haemodynamic compromise. As a result, it is important to look for any RVOT damage after each inflation of the balloon, not only to make the diagnosis but also to define its location and extension. Based on this full description, multiple options are available to solve the problem. In minimal tear, no specific treatment is required. In dissection or flap, a simple bare metal stent can do the job. In extensive lesions at bifurcation or RV level, occluders can be used. Elsewhere, covered stents are particularly helpful to cover a tear. In dramatic situations, it might be necessary to exclude one pulmonary artery with a long, covered stent and send the patient for surgery. New preloaded covered stent systems such as the NuDEL™ system (NuMED, Inc.) might be helpful to deal with such a situation rapidly. Patients with heavily calcified and stenotic homografts have been found to be at risk17. In these patients, some operators advocate either: 1) placing a long covered stent throughout the RVOT without full opening before testing the coronaries; or 2) achieving gradual expansion by sequentially dilating the homografts with balloons of increasing diameter until the diameter of the intended valve implanted is reached18. In our opinion, these two approaches carry either a risk of coronary compression as we have seen coronary compression at RVOT opening as low as 8 mm in diameter (Figure 5), or the same risk of conduit rupture at each inflation as the calcium is mobilised each time. The choice between these approaches is more cultural and operator-dependent. It is, however, important to understand the drawbacks of each technique and to be prepared to treat a conduit rupture, a coronary compression or both in the worst case scenario.
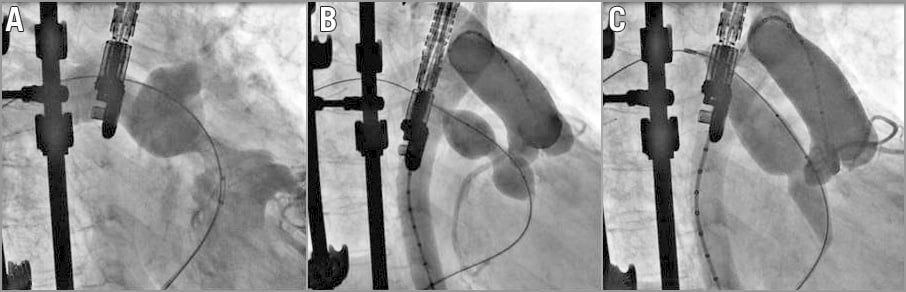
Figure 5. Coronary testing prior to valve implant. A) RVOT injection showing a tight stenosis of the RVOT. B) Inflation of a balloon showing an indentation and a patent right coronary artery proximal to the RVOT. C) Further inflation of a larger balloon showing total occlusion of the RCA at 8 mm RVOT opening.
TECHNICAL MODIFICATIONS FOR CHALLENGING RVOT
Various technical modifications have been described to cover all clinical situations. These techniques were essentially developed for Melody valves.
FOLDED TECHNIQUE19,20 (Moving image 1)
This is very useful for those with short, complex RVOTs, early bifurcation and the potential of coronary artery obstruction by the stent edge. This is carried out by hand-folding the terminal stent struts over a syringe before crimping from inside out at one or both ends. The folded valve should then be crimped on a delivery system without difficulty. Folding reduces the number of rows of the stent which drop from six to five or four, allowing a clear and sharp demarcation of its proximal and distal ends. The length of a CP8Z34 (six rows) expanded on a 22 mm balloon is 24.6 mm. The length of a CP8Z22 (four rows) is 16.7 mm when expanded on a 22 mm balloon, giving a reduction in length of 7.9 mm. Alternatively, when the landing zone is short, an Edwards SAPIEN can be used without modification.
RUSSIAN DOLL TECHNIQUE AND/OR BRANCH PULMONARY ARTERY JAILING FOR ANCHOR IN LARGE PATCHED RVOTS21,22
If the pulmonary arteries (PAs) are large and the RVOT between 25 and 30 mm, then multiple stents with decremental diameters may be deployed with full overlapping in the RVOT region to reduce its size and allow proper seating of the Melody valve. The stent of choice for this method (Russian doll technique) is usually a high-profile stent (CP10Z or CP8Z45 covered stent; NuMED, Inc.). In the presence of PA stenosis, the smallest or stenosed PA branch is used as an anchor for multiple stents and subsequently for Melody valve insertion. When the diameter of a PA branch is <22 mm and the RVOT around 25 mm, stents are deployed from that PA branch all the way to the RVOT with overlapping uncovered stents, thus jailing the opposite PA (PA jailing technique). Uncovered bare metal stents with an open-cell design may be used for this application (Max™ LD 36 mm; ev3/Covidien/Medtronic, Irvine, CA, USA). An overlap of 30% to 50% is important. The Melody valve can then be inserted safely inside the ev3 using an Ensemble 22 mm delivery catheter. If needed, the Melody can be overdilated with a 24 mm balloon3. When the diameter of a PA branch is <22 mm and the RVOT between 25 and 30 mm, both techniques are usually needed for the patient, with multiple covered stents implanted inside the jailing stent prior to Melody valve implantation (Moving image 2). One should notice that these techniques are not now needed with the availability of the 29 mm SAPIEN valve.
DIABOLO SHAPE STENT23
A diabolo-shaped stent is being created using the property of two different stents used simultaneously with one fitted on the second one. A bare metal stent with limited expansion allows a restrictive region in the middle part in stent assembly. A covered stent with more expansion capabilities allows anchoring to larger structures. The choice of stent and balloon is made according to the targeted external and internal diameters. For large diameters, long covered stents (10Z; or Bentley BeGraft aortic stent [Bentley Innomed GmbH, Hechingen, Germany]) and Mega™ LD (S17-26; ev3/Covidien/Medtronic) are preferred. Stent assemblies are crimped over large balloons (BIB or NuCLEUS™ balloons [NuMED, Inc.]; Cristal balloon [Balt, Montmorency, France]). A short Mega LD is then crimped over and on the middle part of the covered stent. These stents are chosen based on the manufacturers’ charts. According to these charts, the Mega LD cannot be expanded over 22 mm in diameter and foreshortening is maximal at this diameter. The balloon is slowly inflated and deflated after full opening. The shape of the created stent is like a diabolo. The extremities anchor to the pulmonary wall while the middle part shelters the valve to be implanted. This technique is particularly useful in large RVOT and can also be used with SAPIEN valves.
ONE-STEP PROCEDURE (Moving image 3)
This technique was developed in order to reduce procedural time and radiation exposure. This is presently the technique of choice in our laboratory in classic cases. Similar to the folded technique, where we have seen that a layer could be added to the Melody without compromising the use of a conventional delivery system, we have developed a technique where bare metal stents (BMS) are crimped over the valve. The Melody valve is hand-crimped over an Ensemble delivery catheter using a conventional technique after rinsing it with sterile fluid. One to three BMS stents (Max LD) are inflated ex vivo using a vascular balloon. The ev3 stent(s) is then manually crimped over the Melody taking care to superimpose each one over the other. The Melody assembly is then loaded as usual. Deployment of the assembly is finally achieved using the conventional technique. It is of note that we have been using the same technique to deliver multiple BMS at the same time in the RVOT prior to PPVI for highly mobile RVOT (or during the Russian doll technique), thus reducing recoil and limiting the risk of stent fracture. Other authors have modified the Ensemble delivery catheter to deliver SAPIEN valves24.
SIDE-BY-SIDE TECHNIQUE
The technique was developed for large patched RVOT not amenable to any of the techniques described previously. Phillips et al reported initially a hybrid strategy where they implanted perventricularly one to three covered stents besides the valved stent in patients with irregular RVOTs25. The covered stents were then filled with a plug to reduce the paravalvular leak. It is of note that a pre-stenting with a Palmaz stent (XD4010 or 5010; Cordis, Cardinal Health, Milpitas, CA, USA) mounted on a 28 or 30 mm NuCLEUS balloon was performed before implantation of a SAPIEN 29 mm and the covered stents. This technique was mainly designed to avoid paravalvular leak and not to stabilise the valve. Based on this experience, we modified our approach to large RVOT and developed a technique called the “side-by-side technique” for patients with an RVOT up to 34 mm not amenable to any of the available valves. This technique is only transcatheter. No surgical access is required. Two Melody Ensemble 22 mm delivery catheters are positioned in the RVOT, one containing a Melody with an ev3 stent over it (as described previously in the one-step procedure), and one with a CP8Z39 covered stent with an ev3 stent over it. The covered stent is firstly deployed. The Melody is then inflated, taking care to maintain the balloon of the covered stent until there is full apposition of the Melody to the wall. Full inflation of the Melody is performed while deflating the balloon of the BMS, thus leading to the crushing of the covered stent. After retrieval of the delivery systems, the lumen of the BMS is filled with an AMPLATZER Vascular Plug II (St. Jude Medical, St. Paul, MN, USA) of an appropriate size.
Post-interventional care and long-term complications
Lifelong aspirin is recommended. Melody valve stent fracture was common before the era of routine RVOT pre-stenting26. Percutaneous valve endocarditis continues to be something of a concern with increasing numbers of cases and reports27-29. It is important to keep in mind that valve endocarditis defies most of the classic features of endocarditis and may be very atypical and catastrophic in presentation. Regular surveillance and monitoring of RVOT gradient is important. Any unexplained increase in gradient should be evaluated thoroughly for stent fracture and endocarditis. Good dental and skin hygiene is of extreme importance. Despite the incidence of endocarditis, overall results of the technique are spectacular and very similar to surgical results30-32.
Conclusion
Percutaneous implantation of a valve in the pulmonary position has been performed with high success rates, low procedure-related morbidity, and excellent long-term results. Multiple modifications have been described over the years to increase the number of candidates. Large patched RVOTs are still tricky to treat, requiring multiple skills. Newer self-expanding valves (Venus P-valve [Venus MedTech, Hangzhou, China]; Harmony™ [Medtronic]) will facilitate PPVI in that subgroup of patients33,34. Preliminary results have been excellent but with limitation of the indications to native RVOT with parallel borders (tubular) and diameter not exceeding 32 mm because these self-expanding devices require oversizing from 2 to 5 mm. There remains, however, room for further research and newer devices to expand the present indications to all RVOT geometry and anatomy, and to all patients irrespective of their age and body size.
Conflict of interest statement
The author has no conflicts of interest to declare.
Supplementary data
Moving image 1. Patient with a short RVOT who received a pre-stenting with a Max LD 26 mm followed by implantation of a folded Melody valve.
Moving image 2. Patient with patched RVOT. Multiple BMS are being implanted starting from the left pulmonary artery down to the main pulmonary artery. A CP covered stent is then placed to apply the BMS to the pulmonary wall. Finally, a Melody valve is inserted with good results.
Moving image 3. Patient who received a Melody valve and BMS at the same time (one-step procedure).
Supplementary data
To read the full content of this article, please download the PDF.
Patient with a short RVOT who received a prestenting with a Max LD 26 mm followed by implantation of a folded Melody valve.
Patient with patched RVOT. Multiple BMS are being implanted starting from the left pulmonary artery down to the main pulmonary artery. A CP covered stent is then placed to apply the BMS to the pulmonary wall. Finally, a Melody valve is inserted with good results.
Patient who received a Melody valve and BMS at the same time (one-step procedure).

