
Abstract
Aims: The aim of the study was to evaluate the midterm safety and efficacy of a self-expanding valve (Venus P-valve) in the treatment of patients with pulmonary regurgitation and a native right ventricular outflow tract (RVOT) in China.
Methods and results: Patients who had moderate or severe pulmonary regurgitation after surgical repair of the RVOT with a transannular or RVOT patch were included in the study. Fifty-five patients (67% female; average age 28.7±12.4 years) from six different hospitals in China were enrolled. The procedure success rate was 98.2%. In the one failure, the patient experienced valve dislodgement two days after the procedure. During the 12-month follow-up, two patients died, one due to infective endocarditis. Three other patients developed infective endocarditis. Two patients developed atrial flutter, and one patient had a pulmonary embolism. Echocardiography examinations at 12 months showed that two patients had mild pulmonary regurgitation, and 19 patients had trace pulmonary regurgitation. No paravalvular regurgitation occurred. The mean peak pulmonary gradient was 16.3±7.4 (range 4-38) mmHg. Compared with the baseline data, the right ventricular end-diastolic volume index (RVEDVI) was reduced from 137.6±15.8 mL/m2 to 83.9±16.0 mL/m2 (p<0.001), and the New York Heart Association (NYHA) class was significantly improved (p<0.01).
Conclusions: The one-year results of the China Venus P-valve study show considerable promise for a hitherto unmet need in patients with pulmonary regurgitation and an enlarged native RVOT.
Abbreviations
IE: infective endocarditis
MPA: main pulmonary artery
MRI: magnetic resonance imaging
MSCT: multislice computed tomography
PPVI: percutaneous pulmonary valve implantation
RVEDVI: right ventricular end-diastolic volume index
RVOT: right ventricular outflow tract
Introduction
Percutaneous pulmonary valve implantation (PPVI) is a promising technology for treating patients with chronic pulmonary regurgitation following surgical reconstruction of the right ventricular outflow tract (RVOT). To date, the clinical practice of PPVI has been largely limited to the use of two balloon-expandable systems, namely the Melody® valve (Medtronic, Minneapolis, MN, USA) and the SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA). Both valves are recommended for implantation only in RVOTs composed of homografts or conduits1. However, most patients in China undergo surgical reconstruction of the RVOT using a transannular patch technique2, which results in a dilated and distensible RVOT that usually exceeds the diameter of available percutaneous valves. Thus, there is a great demand for a valve that can be implanted in individuals with a large native RVOT in China.
The Venus P-valve (Venus Medtech, Hangzhou, China) is a self-expanding percutaneous valve designed to address this problem. The first implantation of a Venus P-valve was reported by our group in 20143. Subsequently, more studies have been reported by other groups4-6. Since 2013, a multicentre prospective observational study has been performed in China (NCT02590679). The objective of the study was to evaluate the midterm safety and efficacy of PPVI using the Venus P-valve in patients with pulmonary regurgitation and a native RVOT in China.
Methods
STUDY DESIGN
This non-randomised, prospective, multicentre, early feasibility study was conducted at six centres in China (Zhongshan Hospital of Fudan University, Fuwai Hospital, West China Hospital of Sichuan University, Shanghai Chest Hospital, Shanghai Children’s Medical Center, and Xijing Hospital of the Fourth Military Medical University). The primary effective endpoint was right ventricular end-diastolic volume index (RVEDVI) at six months. The primary safety endpoints were the occurrence of death or reoperation (valve- and/or procedure-related) at 12-month follow-up. Clinical assessments, chest X-rays, electrocardiography and transthoracic echocardiography were performed at baseline and at follow-up periods of 1, 3, 6 and 12 months. Multislice computed tomography (MSCT) and cardiac magnetic resonance imaging (MRI) were performed at baseline and six months.
The following inclusion criteria were applied: (1) moderate to severe pulmonary regurgitation following surgical repair of congenital RVOT obstruction with a transannular or RVOT patch, (2) age of 10 years or older and 60 years or younger, (3) weight ≥18 kg, (4) pulmonary annulus diameter of 14-31 mm, (5) RVOT and main pulmonary artery (MPA) length ≥20 mm, (6) symptoms (New York Heart Association [NYHA] cardiac function class ≥2) related to pulmonary regurgitation or a pre-existing condition, such as RVEDVI ≥150 ml/m2, progressive right ventricular systolic dysfunction, progressive tricuspid regurgitation (at least moderate), RVOT obstruction with right ventricular systolic pressure ≥80 mmHg, or sustained atrial/ventricular arrhythmias, and (7) providing signed informed consent. The following exclusion criteria were applied: (1) pre-existing pulmonary artery stenosis or previous artificial pulmonary valve implantation, (2) severe chest wall deformity (funnel chest, etc.), (3) acute decompensated heart failure, (4) active infection or endocarditis requiring antibiotic therapy, (5) leukopaenia (white blood cell <3,000/mm3), (6) acute or chronic anaemia (haemoglobin <9 g/L), (7) platelet count <5,000/mm3, (8) no possible access route for delivering a Venus P-valve to the RVOT as judged by a researcher before the procedure, (9) known allergy to aspirin or heparin, and (10) positive urine or serum pregnancy test in female subjects. The study was approved by the Chinese Food and Drug Administration and the local institutional review board at each participating institution. For all study participants, informed consent was obtained from the patients or their relatives.
DEVICE DESCRIPTION
The Venus P-valve is a self-expanding percutaneous pulmonary valve. It has been described in detail in previous studies3-6. In brief, it is composed of a nitinol support frame and a trileaflet porcine pericardial tissue valve with a 22-24 Fr delivery catheter. The entire stent is covered (except for the distal cells) with porcine pericardial tissue. The proximal and distal ends are flared to secure anchoring at the pulmonary artery bifurcation end and the RVOT. The stent valve diameters range from 16 to 32 mm (in 2 mm increments), with each diameter available in 20, 25 and 30 mm straight section lengths (Figure 1).
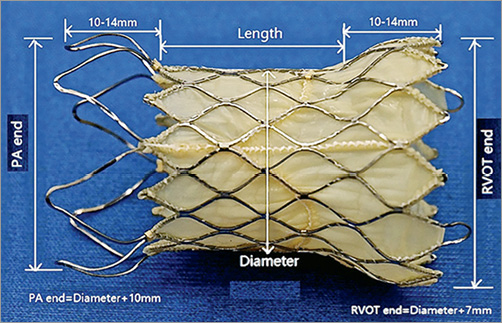
Figure 1. Venus P-valve sizing. The Venus P-valve is a bespoke prosthesis, tailored to individual patient anatomy, made to order based on baseline MSCT, with 10 different sizes utilised in the study. The valve length is defined as the length of the straight section. The valve diameter is the diameter of the straight section (prosthesis annulus). The diameter of the PA end is 10 mm larger than the valve diameter, and the RVOT end is 7 mm larger. The length of the flared segments depends on the width of the valve: 10 mm for the smaller valves and 14 mm for the larger valves.
PROCEDURE
All procedures were performed with the patients under general anaesthesia in a catheterisation lab or hybrid room. Appropriate anticoagulation was performed with heparin, and intraprocedural antibiotics were administered. All procedures were guided by fluoroscopy or angiography and transoesophageal echocardiography. The access route was the right femoral vein. Right heart catheterisation and RVOT angiography were performed. The length of the RVOT, MPA and the pulmonary annulus diameter were measured. The Lunderquist® extra stiff guidewire (Cook Medical, Bloomington, IN, USA) was advanced into the distal pulmonary artery. Then, a 34 mm compliant balloon catheter was delivered to the RVOT and MPA over the guidewire. Another pigtail catheter was placed into the aortic root. Unselective coronary angiography while the balloon was fully inflated was performed to assess the probability of compression of the valve stent on the coronary artery. The diameter of the compliant balloon was measured as a reference for selecting the sizes of the Venus P-valve. If no contrast leaked to the RVOT after pulmonary angiography when the balloon can slightly slip in the RVOT, the balloon diameter was considered the right size after inflation. Generally, a valve diameter 3-5 mm larger than the measurement obtained from balloon sizing was chosen for implantation. The valve lengths were selected based on the RVOT and MPA length measured by RVOT angiography. After being loaded, the valve was sent to the RVOT and MPA along the guidewire. After the distal flare was exposed in the left pulmonary artery, the system was slowly withdrawn to allow the flare to expand fully in the distal MPA. Repeat RVOT angiography was performed to ensure that the valve was positioned correctly. After confirming that the valve was in the ideal position, it was completely released, and the delivery system was pulled out. Repeat haemodynamic assessments along with MPA angiography and echocardiographic assessments of the valve were performed. Catheter images of one typical case during the Venus P-valve implantation procedure are shown in Figure 2. Patients were generally given 3,000 IU heparin during the procedure. Oral aspirin 100 mg per day was given for six months after the procedure.
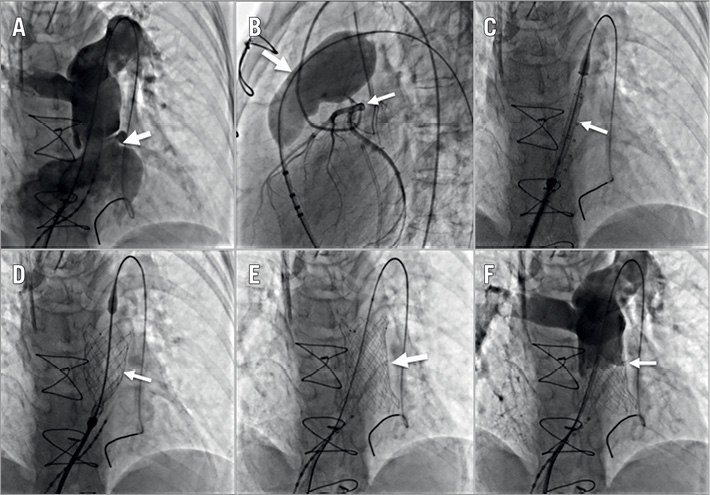
Figure 2. Catheter images during the Venus P-valve implantation procedure. A) Cine-angiography showing severe pulmonary regurgitation (PR) (arrow). B) Balloon (large arrow) sizing of the RVOT and MPA and a cine-angiography of the coronary arteries (small arrow). C) Venus P-valve delivery in the PA (arrow). D) Venus P-valve partially deployed (arrow). E) Venus P-valve completely deployed in the MPA (arrow). F) Cine-angiography showing no residual PR (arrow).
STATISTICS
Continuous variables and categorical variables are presented as the mean value±SD (and range) and percentages, respectively. Values of continuous variables at baseline and at the six-month follow-up were compared using a paired Student’s t-test or the Wilcoxon rank-sum test. All p-values were based on two-sided tests and were considered to be statistically significant at p<0.05. Statistical analyses were performed using SPSS, Version 13.0 software package (SPSS Inc., Chicago, IL, USA).
Results
BASELINE CHARACTERISTICS
A total of 55 patients from six hospitals in China were enrolled in the study. The average age was 28.7±12.4 years. Five patients had moderate pulmonary regurgitation and 50 patients had severe pulmonary regurgitation. Patient characteristics are shown in Table 1.
PERIOPERATIVE RESULTS
The valve sizes and numbers used in the patients are shown in Table 2; they were made to order based on careful preprocedural MSCT sizing. The procedure success rate was 98.2% (54/55). The fluoroscopy time was 22.86±7.22 minutes. All patients were discharged with successful pulmonary valve replacements except for one patient who had valve dislodgement to the right ventricle two days after the procedure and who underwent surgery. The preoperative computed tomography images showed that the patient had an inverted cone-shaped RVOT and MPA and a single left pulmonary artery (Figure 3). The valve was released at the RVOT and MPA but migrated proximally to the RVOT two days later. The valve was moved to the pulmonary annulus and sutured at this site. Four patients had unexplained fever after the procedure. Their body temperatures ranged from 37.5-39°C. The blood cultures were negative for these patients. After three to five days of antibiotics, their fevers were relieved. One patient had a femoral haematoma.
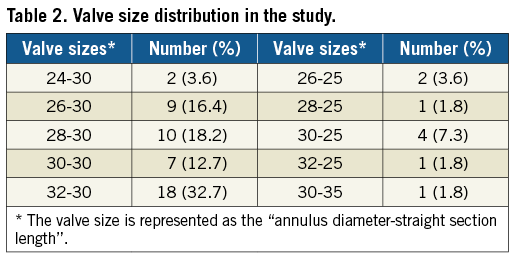
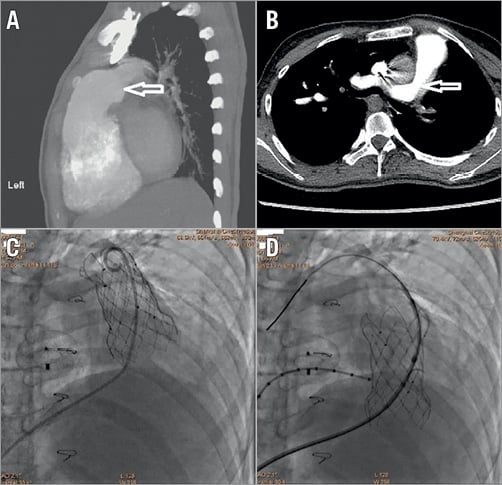
Figure 3. CT and fluoroscopic images of the patients with valve migration. Lateral (A) and horizontal (B) CT images show the inverted cone-shaped RVOT and MPA (arrow) and single left PA. C) Fluoroscopic image showing that the valve was released at the RVOT and MPA. D) Fluoroscopic image showing subsequent valve migration into the RVOT.
TWELVE-MONTH FOLLOW-UP
No patients were lost to follow-up at 12 months. One patient died following a road traffic accident four months after the procedure, and one died due to infective endocarditis (IE) three months after the procedure. There were three other cases of infective endocarditis occurring at 2, 3 and 11 months after the procedure. One patient was treated with surgical pulmonary replacement and the other two patients were treated medically with six weeks of antibiotics. Two patients developed atrial flutter: one was electrically cardioverted, and the other underwent catheter radiofrequency ablation. Both patients maintained sinus rhythm throughout the subsequent follow-up period. One patient had a pulmonary thrombosis embolism which was relieved by intravenous urokinase. Echocardiography examinations at 12 months showed that two patients had mild pulmonary regurgitation, and 19 patients had trace pulmonary regurgitation (Figure 4A). Otherwise, all remaining patients did not have any pulmonary regurgitation, and no cases of paravalvular regurgitation occurred. The patients’ peak pulmonary gradient was 16.3±7.4 (range 4-38) mmHg at 12 months and just two of the patients had peak gradients greater than 30 mmHg (Figure 4B). The NYHA class was significantly improved at six months and 12 months as compared with baseline (p<0.01) (Figure 4C). The RVEDVI measured by MRI was reduced from 137.6±15.8 mL/m2 at baseline to 83.9±16.0 mL/m2 at six months (n=50, p<0.001) (Figure 4D). No stent fractures occurred as shown by serial chest X-ray examinations.
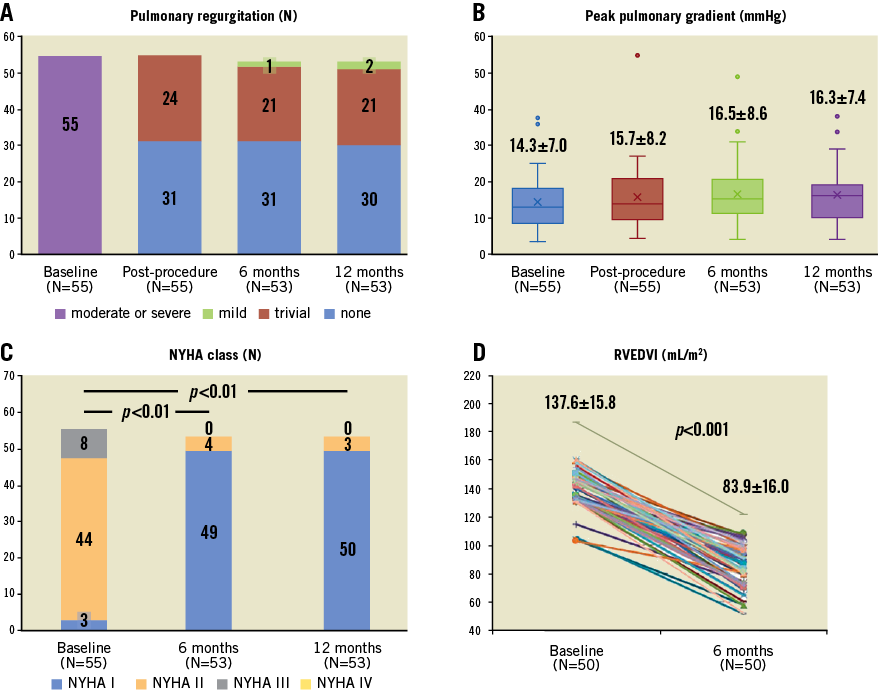
Figure 4. Pulmonary valve haemodynamic parameters, NYHA function and RVEDVI at baseline and follow-up.
Discussion
To the best of our knowledge, this study is the largest-scale clinical study of a self-expanding percutaneous pulmonary valve. The results from this multicentre study show that it is feasible and effective to implant the self-expanding percutaneous Venus P-valve in the native RVOT of patients with chronic severe pulmonary regurgitation following previous transannular patch.
Currently, there are two available percutaneous pulmonary valves (Melody and SAPIEN), which are both balloon-expandable valves that are recommended only for RVOTs composed of homografts or conduits1. They have also been tested in a relatively small native RVOT after a stent was pre-implanted to transform the native RVOT into a rigid conduit7,8. However, for native RVOTs larger than 27 mm, these two systems are not large enough to maintain the stable valve position within the dilated native RVOT, even when using the pre-stenting approach9. Most Chinese patients undergo surgical RVOT repair using a transannular patch technique2. Therefore, their RVOTs are often severely enlarged and so balloon-expandable systems are commonly not suitable for Chinese patients. The self-expanding percutaneous Venus P-valve was thus developed to meet this critical need. Recently, another self-expanding percutaneous pulmonary valve, the Harmony™ valve (Medtronic), was also developed to address this unmet need10. It has been studied in a 20-patient short-term follow-up study thus far. There are two other advantages of the Venus P-valve as compared with available balloon-expandable valves. First, pre-stenting is not needed for the Venus P-valve, even in native RVOT patients. Second, the valve can be easily positioned. The valve is long and its ends are flared, and thus precise positioning is not required. During the releasing of the valve, the position of the valve can be adjusted.
ACUTE OUTCOMES
In this study, all patients had native RVOTs and pulmonary regurgitation following surgical correction of the RVOT. The mean pulmonary annulus diameter, based on echocardiography measurement, was 23.4±4.4 (16.2-30.0) mm, and the mean RVOT diameter was 30.4±5.7 mm. Many patients were anatomically unsuitable for the balloon-expandable valves commercially available. Despite challenging anatomy, favourable haemodynamics were achieved. This prosthesis is notable for the large number of sizes available (Table 2). The Venus P-valve is a bespoke prosthesis, tailored to individual patient anatomy, made to order based on baseline MSCT, with 10 different sizes utilised in the study (Figure 1). Over half (31/55) of the patients were implanted with either 30 or 32 mm valves (Table 2). The average baseline RVEDVI was 137.6±15.8 mL/m2 and, due to severe symptoms, many patients were treated despite an RVEDVI far lower than that which would generally be considered for pulmonary valve replacement (150 ml/m2)11.
Our initial success rate using the Venus P-valve was 98.2%. One patient had proximal migration to the right ventricle two days after the procedure and underwent surgical treatment. This case indicated that the inverted cone-shaped RVOT and MPA (Figure 3) may be not suitable for Venus P-valve implantation. This complication has also been observed with the Medtronic Harmony valve, whose initial feasibility study reported proximal migration in one patient during delivery system removal, and surgical explantation of the device in two additional patients10.
ONE-YEAR OUTCOMES
During the 12-month follow-up, two patients died. No stent fracture was observed, a complication frequently seen with the Melody valve without pre-stenting12. There were four cases (7.3%) of IE in the present study, highlighting that this as a notable but previously recognised complication of PPVI13. After Melody PPVI, the reported incidence of IE is 5.7% (95% confidence interval [CI]: 2.9% to 11.4%) per person-years14. A meta-analysis reported a rate of IE following PPVI of 4.9%, usually occurring within nine months following the PPVI15. The reason why the rate of IE was high in our study may be because antibiotic prophylaxis following surgery and percutaneous procedure including PPVI in China is not so strict. A recently reported experience with more than 10-year follow-up with the Melody valve suggested a male predominance of IE following PPVI. It advocated stricter measures with antibiotic prophylaxis following PPVI as well as aggressive education of adolescent patients and their families for dental and skin hygiene16.
Echocardiography examinations at 12 months found that the valve function was still well maintained. Neither moderate nor severe pulmonary regurgitation occurred. Most of the patients had a low peak pulmonary gradient during the follow-up. The patients’ right ventricles were significantly remodelled, and their cardiac function was improved. The RVEDVI on MRI was reduced from 137.6±15.8 mL/m2 at baseline to 83.9±16.0 mL/m2 at six months after the procedure. NYHA class was improved. Together, these results show that the Venus P-valve was effective in treating patients with pulmonary regurgitation in an enlarged native RVOT. The results appear similar to the early results obtained using the Melody and SAPIEN systems12,17,18 in RVOT homografts or conduits.
Limitations
This study is, to date, the largest safety and efficacy study for a self-expanding PPVI. However, there were some limitations. First, our sample size was small. Second, our follow-up time was short. Further studies with a larger patient population and a longer follow-up time are underway in Asia, Europe and South America to evaluate the safety and durability of the Venus P-valve more comprehensively.
Conclusions
The one-year results of the China Venus P-valve study have shown considerable promise for a hitherto unmet need in patients with pulmonary regurgitation and an enlarged native RVOT. Further studies with longer follow-up are required to confirm the safety and durability of the device.
| Impact on daily practice The one-year results of the China Venus P-valve study were acceptable, indicating that the Venus P-valve is a promising device for treating patients with pulmonary regurgitation and an enlarged native RVOT. |
Funding
This study was supported by Venus Medtech, Inc., Hangzhou, China.
Conflict of interest statement
H. Jilaihawi is a consultant to Venus Medtech. The other authors have no conflicts of interest to declare.

