
Modern surgery and perioperative treatment have markedly improved long-term survival for children with congenital heart disease (CHD), even those with more complex defects. However, when they reached adulthood, previously unknown problems were revealed with the necessity of multiple surgical procedures over the course of time. Right ventricular outflow tract (RVOT) dysfunction1,2, associated with significant morbidity and mortality3, represents a major challenge for the field.
The introduction of percutaneous pulmonary valve implantation (PPVI) by Bonhoeffer et al4 marked a revolution in the management of these patients. Its safety and efficacy have been proven in various studies since then5. Combined with an enhanced understanding of the underlying pathophysiologic mechanism, evolving interventional techniques suggest an earlier, somehow more aggressive, treatment of RVOT dysfunction, before occurrence of irreversible ventricular remodelling and dysfunction.
Current interventional options demand certain morphological requirements for safe and effective implantation. Both currently available valves (Melody™ [Medtronic, Minneapolis, MN, USA], and SAPIEN XT/S3 [Edwards Lifesciences, Irvine, CA, USA]) are designed and approved for patients with a conduit or prosthetic valve in the pulmonary position. There are several reports of successful utilisation of Edwards SAPIEN valves in native or patched RVOT with the aid of bare metal stents6,7, as long as the implantation site diameter does not exceed 29 mm. Consequently, about 2/3 of all patients with native or patch-expanded dilated RVOT are excluded from current percutaneous treatment options.
The need for a device dedicated to dilated native RVOTs was recognised early on with the initial description of PPVI. Eighteen years on and ten years after the first successful implantation of a PPVI device for dilated RVOT in 20108, there is still no established transcatheter treatment option for these patients (Figure 1).
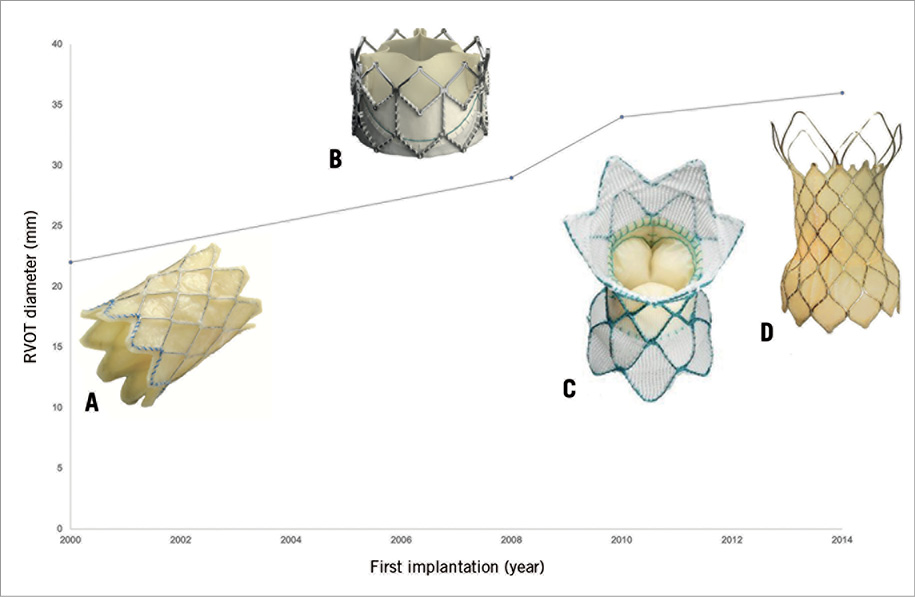
Figure 1. Evolution of PPVI. A) Melody valve (Medtronic), balloon-expandable, max. diameter 22 mm. B) SAPIEN XT valve (Edwards Lifesciences), balloon-expandable, max. diameter 29 mm. C) Harmony® valve (Medtronic), self-expanding, inflow diameter 34 mm, outflow diameter 42 mm. D) Venus P-valve (Venus Medtech), self-expanding, max. diameter 36 mm.
The more recently introduced Venus P-valve® (Venus Medtech, Shanghai, China) represents another PPVI device for large RVOTs. It is a self-expanding transcatheter heart valve, consisting of a trileaflet porcine pericardial tissue valve, mounted on a nitinol stent frame9. Its design features proximal (completely tissue covered) and distal (open wire frame) flares to anchor the valve in the RVOT and PA bifurcation, respectively. Measured by the straight middle segment, diameters from 18 to 36 mm (in 2 mm increments) resulting in a total valve length of 20 to 35 mm (in 5 mm increments) are available.
Following encouraging early clinical experience9,10, Morgan et al present a retrospective multicentre collection of implants in the current issue of EuroIntervention11.
In a total of 38 patients at 11 international centres, PPVI using the Venus P-valve was performed. Successful valve implantation was achieved in 97% of cases and is therefore in line with early Melody12 and Edwards SAPIEN data6. Interventions resulted in effective reduction of pulmonary regurgitation.
Unlike the procedural approach to Melody and SAPIEN implantation in the pulmonary position, no pre-stenting prior to Venus P-valve implantation was performed. While no major valve embolisation occurred, there were two cases of valve migration immediately after implantation, resulting in surgical retrieval.
The rate of stent fractures was higher than in comparable reports using the Melody valve13, especially if pre-stenting, today considered as common practice in most centres, was performed. Since previous studies have identified native or patch-extended RVOTs as risk factors for stent fractures, this higher rate might somehow be expected in these implant environments and highlights the importance of assessing and meticulously reporting these events. As all fractures affected the proximal flare, one might speculate whether future design evolution could address possible construction weaknesses in this area. Whether pre-stenting might be another option also remains elusive. However, the authors themselves question the utilisation of the Venus P-valve after prior side branch stenting as noticeable difficulties were experienced in advancing and safely deploying the assembly under these conditions. More data with longer and, most importantly, complete follow-up might identify further risk factors in these patients with native RVOTs, eventually helping to avoid these complications.
After the introduction of PPVI almost two decades ago, this technique was introduced into clinical routine for the treatment of RVOT dysfunction at experienced centres. The success of PPVI using the Melody device and, in part, the SAPIEN device is a consequence of a shared learning curve, careful patient selection including clinical and morphological specifics, and close and complete assessment of procedural success both early and during follow-up. This has led to changes in technology and technique, thereby constantly improving the safety and effectiveness of PPVI.
Morgan et al should be congratulated for their work, describing a promising new device with the potential to extend PPVI to the large cohort of patients with native and patch-extended RVOT with larger diameters. To live up to this promise, meticulous, multimodal, continuous and complete assessment of procedural results of the Venus P-valve comparable to those of the Melody programmes represents a prerequisite for future activities, especially in the field of congenital heart disease, in which large randomised trails are difficult to perform and in which almost every patient’s anatomy and clinical situation is unique.
Conflict of interest statement
The authors have no conflicts of interest to declare.

