
Abstract
Aims: Percutaneous pulmonary valve implantation (PPVI) is used to treat patients with dysfunctional pulmonary valve conduits. Short- and longer-term results from multiple trials have outlined haemodynamic improvements. Our aim was to report the long-term results, including quality of life, from a multicentre trial in Europe and Canada.
Methods and results: From October 2007 to April 2009, 71 patients (24 female; median age 19.0 [IQR: 14.0 to 25.0] years) were enrolled in a prospective cohort study. PPVI was performed successfully in 63 patients. At five-year follow-up four patients had died. Moderate and severe pulmonary regurgitation were completely resolved in all except one patient, who needed re-PPVI. Outflow tract obstruction improved significantly from a mean pressure gradient of 37.7±12.1 mmHg before PPVI to 17.3±9.7 mmHg at five-year follow-up; however, 11 patients needed treatment for restenosis. The EQ-5D quality of life utility index and visual analogue scale scores were both significantly improved six months post PPVI and remained so at five years.
Conclusions: Five-year results following PPVI demonstrate resolved moderate or severe pulmonary regurgitation, improved right ventricular outflow tract obstruction, and improved quality of life.
Introduction
Percutaneous pulmonary valve implantation (PPVI) is used to treat patients with dysfunctional right ventricular to pulmonary artery conduits1.
While multiple studies have reported improved, sustained haemodynamics and exercise capacity after PPVI, data on long-term results are limited2-10. Cheatham and colleagues reported sustained improvement in mean right ventricular outflow tract (RVOT) gradient and low five-year freedom from reintervention and explantation11. One single-centre study reported that five-year freedom from surgery was 90% and that freedom from reintervention was 68%. The degree of pulmonary regurgitation (PR) was also reduced12. The impact of PPVI on quality of life (QOL) is fairly unknown beyond the first six months13.
Patients and methods
STUDY DESIGN
This non-randomised, prospective, multicentre cohort study enrolled patients with dysfunctional RVOT conduits at seven sites in Europe and Canada. Inclusion and exclusion criteria are described in the Online Appendix. The study was sponsored, designed, and funded by Medtronic (Minneapolis, MN, USA), which was also responsible for site selection, data monitoring and management, and all statistical analyses. The study was approved by the institutional review board/ethics committee at each site. Each patient provided written, informed consent, and the study was conducted in accordance with the Declaration of Helsinki14.
OUTCOMES
The primary objective of the study was clinical performance over five years, as measured by the composite endpoint of freedom from PPV dysfunction, explant, reintervention, stent fracture, and device- or procedure-related death. PPV dysfunction was defined as either a mean continuous wave (CW) Doppler gradient >40 mmHg, RV to pulmonary arterial (RV-PA) peak-to-peak catheter gradient >40 mmHg, moderate or severe PR by echocardiography or angiography, or PR fraction >30% by magnetic resonance imaging.
Secondary outcomes included procedural success, device- and procedure-related mortality and morbidity, haemodynamic performance, and QOL. Procedural success was defined as the PPV implanted within the desired location, RV-PA peak-to-peak gradient <35 mmHg and no more than trivial PR via angiography after PPVI, and freedom from explantation at 24 hours post implant. Adverse events were classified as serious (SAE) if they led to death, serious deterioration in the patient’s health, or foetal distress, death, congenital abnormality, or birth defect.
PROCEDURE DETAILS
Enrolled patients underwent cardiac catheterisation under general or local anaesthesia. The RV/aortic pressure ratio and peak-to-peak RV-PA systolic gradient were both calculated. To assess the suitability of the RVOT conduit for PPVI, angiography was performed to measure the narrowest conduit diameter at the site of intended implantation. The implanter determined the size of angioplasty balloons, balloon waist size, post-dilation, and the need for concomitant procedures, including pre-stenting. Only Melody® valves (a stented bovine jugular vein prosthesis; Medtronic, Minneapolis, MN, USA) were implanted.
FOLLOW-UP EVALUATION
Clinical assessment (NYHA class), QOL assessment, transthoracic echocardiography (TTE), and chest radiography (frontal and lateral) were performed at discharge, six months, one year, and annually thereafter up to five years post implantation at each site. Chest radiographs were analysed and classified in each centre for dislocation and stent fracture15. Major stent fractures were defined as those that required intervention.
ECHOCARDIOGRAPHY
TTE was used to assess haemodynamic performance before and during the five years after implantation, through measurements of mean RVOT gradient by CW Doppler and PR by colour Doppler. Severity of PR was classified as none, trivial, mild, moderate, or severe16. All echocardiographic data were site-reported.
QUALITY OF LIFE
QOL assessments were performed using the EQ-5D pre-implant, at discharge, and at pre-specified follow-up visits. Only patients at least 15 years old completed the questionnaire. Results are reported as a single utility index from the five questions using the European Value Set and time-trade-off method and as a visual analogue scale (VAS) rating. Further details are included in the Online Appendix.
STATISTICAL ANALYSIS
Descriptive statistics were used for procedural and clinical outcomes and echocardiographic data. Results are presented as number (%) or mean±SD. Kaplan-Meier analyses were performed for time-to-event data, and the standard error for freedom from an event was calculated using Peto’s method.
Differences between baseline and follow-up were tested with Wilcoxon signed-rank tests for paired data. Differences between cohorts were tested with Fisher’s exact test for categorical variables or Wilcoxon rank-sum tests for continuous variables. To identify factors associated with each outcome, univariate analyses were run on several covariates using logistic regression. Factors considered were gender, institution, conduit type, age (≥22 years vs. ≤21 years), primary indication, number of open heart surgeries, original diagnosis, and pre-stenting. A log-rank test was performed to test if freedom from PPV dysfunction differed across several variables. A linear relationship between the EQ-5D utility index and baseline mean RVOT gradient was assessed, and the slope was tested with the F-test; p<0.05 was considered significant. All statistics were calculated with SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
PATIENTS
Seventy-one patients were enrolled and catheterised between October 2007 and April 2009. The enrolled cohort was predominantly male (n=47, 66.2%); mean age was 21.6±10.6 years (range 8-59). Original diagnoses included various congenital heart defects, predominantly tetralogy of Fallot. Indication for PPVI was almost balanced for pure stenotic, pure regurgitant, and mixed conduit dysfunction. Baseline demographics are listed in Table 1.
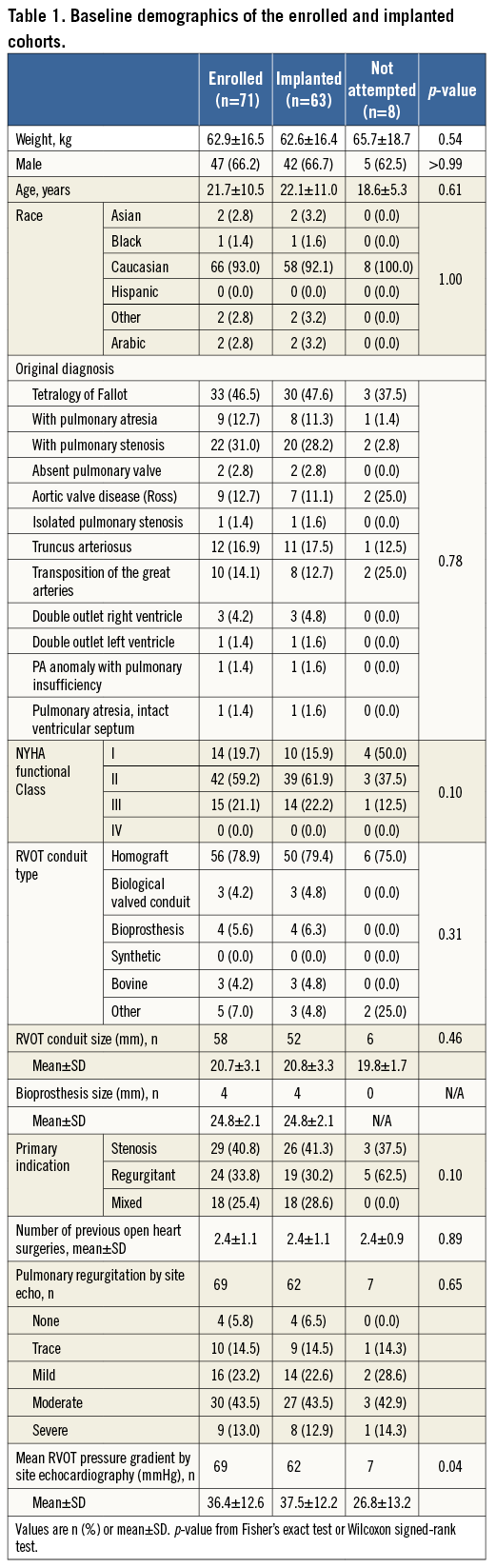
PROCEDURAL OUTCOMES
The majority of catheterisations were via the femoral vein (n=68, 95.8%) with three via the jugular vein (4.2%) in patients with bilaterally occluded femoral veins. Mean procedure time was 168.2±68.6 minutes. The RVOT conduit was stented before PPVI in 81.7% of patients (Table 2).
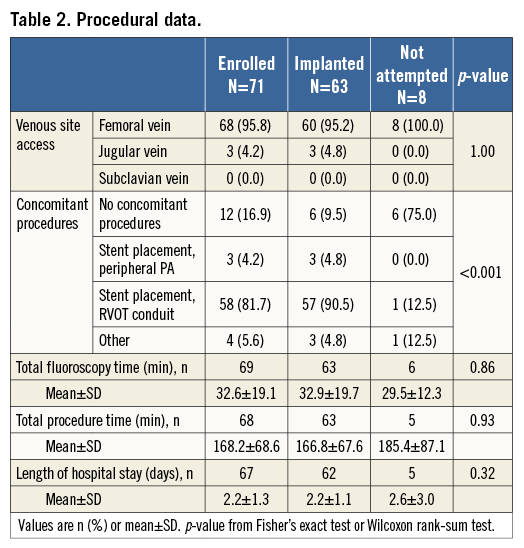
Sixty-three patients were ultimately implanted with the Melody valve (Figure 1). An implant was not attempted in eight patients, due to risk for coronary artery compression (n=2), RVOT conduit not suitable for implant (n=3), desired result achieved with bare metal stenting (n=1), asymptomatic primary stenosis (n=1), and unstable sizing balloon (n=1). Patients whose procedure was aborted had a significantly lower RVOT mean gradient than those who were implanted (p=0.04) (Table 1).
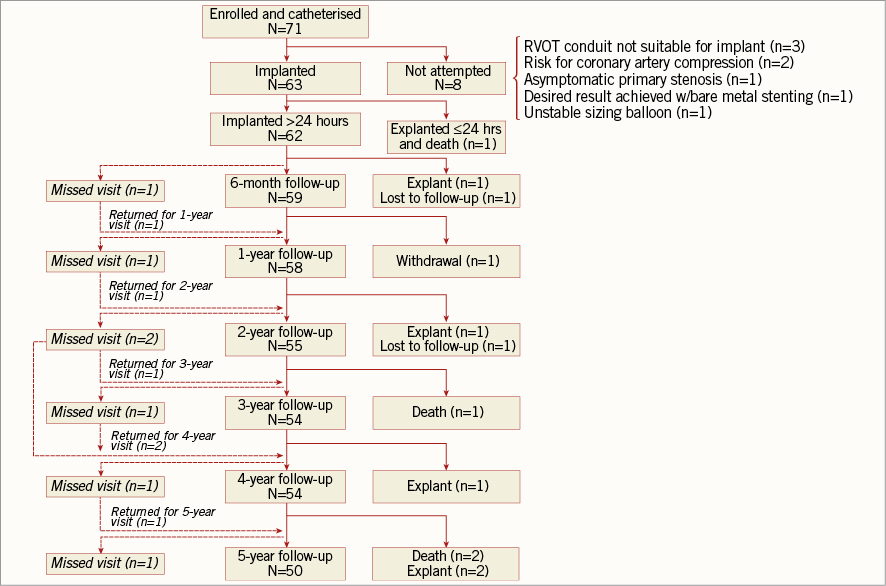
Figure 1. Patient disposition flow chart.
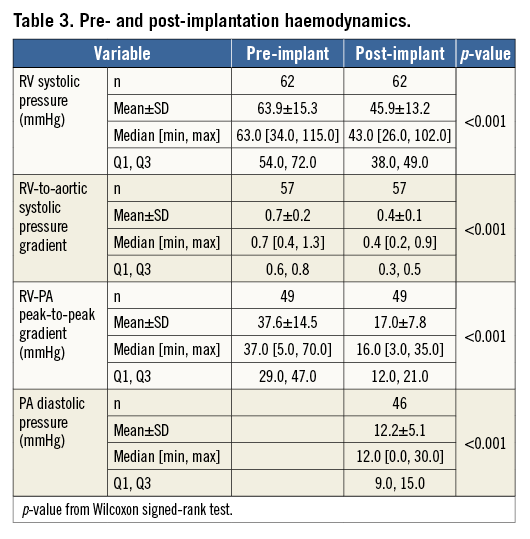
In all 63 patients the PPV was fixed at the desired position. RV-PA peak-to-peak pressure gradient was lowered below 35 mmHg in 60 patients. RV-PA gradient was not assessed in two patients, but their pressure gradients at discharge were 11 and 14 mmHg, respectively, resulting in 62 (98.4%) patients with a successful reduction of RVOT pressure gradient. For 49 patients with paired data, the RVOT peak-to-peak pressure gradient was reduced following implantation (p<0.001) (Table 3). Other acute haemodynamic outcomes are reported in Table 3.
Angiographic PR after PPVI was absent or trivial in 56 of 60 (93.3%) patients. Three patients did not have PR assessed angiographically, but all had no or trivial PR on echocardiography at six months, yielding a total PPVI success rate of 93.7% (59/63).
One patient, who had balloon testing with a 16 mm balloon and pre-stent implantation with an 18 mm balloon without complication, experienced coronary artery compression after PPVI with an 18 mm balloon. The bioprosthesis was explanted within 24 hours, as coronary artery compression caused a myocardial infarction. The patient died 16 days after implant of left-sided heart failure and thus was not included in subsequent analyses.
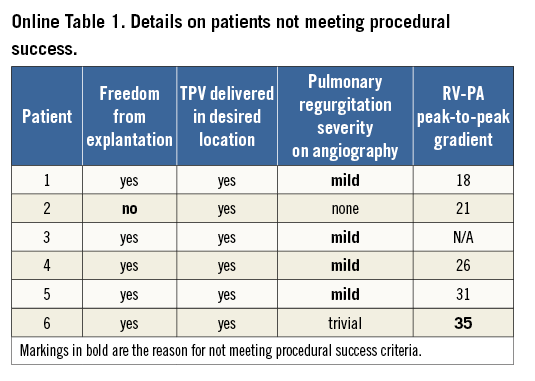
Procedure success was achieved in 57 of 63 (90.5%) patients, but six patients did not meet the criteria for success (Online Table 1). None of the factors analysed by logistic regression was significantly associated with procedural success.
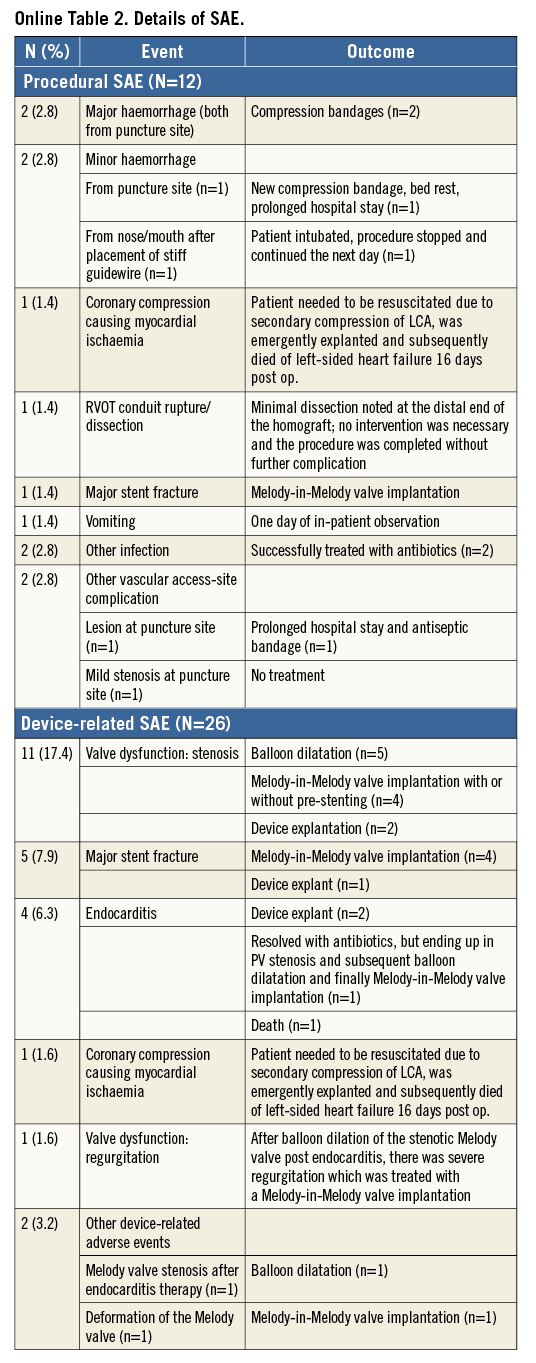
Twelve patients (16.9%) experienced procedure-related SAE (Online Table 2). Except for the deceased patient and one patient with mild stenosis at the puncture site, all adverse events were addressed without sequelae.
LONG-TERM CLINICAL OUTCOMES
Of the 62 patients without explantation within the first 24 hours, the mean follow-up length was 55.7±13.6 months, yielding 288 total follow-up years.
Overall there were four deaths: one as a consequence of the procedure within the first month as discussed above, one due to endobronchial bleeding from a bronchial artery malformation two years after PPVI, one due to an unexplained sudden cardiac event four years after implantation, and one due to septic shock, probable endocarditis, four years after PPVI. Freedom from all-cause death was 98.4% at one year and 93.2% at five years (Online Figure 1).
At baseline, 21.1% of patients were in NYHA Class III; this was reduced to 1.8% at six months (p<0.001) and 8.2% at five years (Figure 2). Logistic regression of NYHA Class I/II versus III/IV at six months and five years post PPVI identified no significant factors impacting on NYHA class.
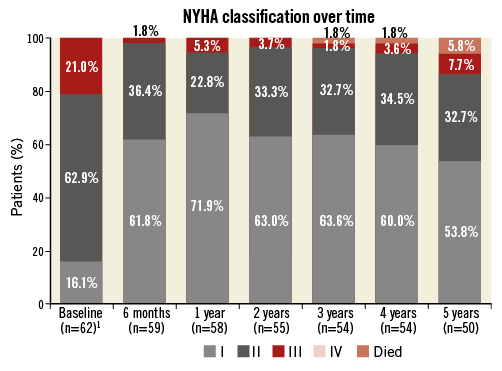
Figure 2. NYHA classification over time. ¹ Number of surviving patients.
HAEMODYNAMIC OUTCOMES
Mean RVOT pressure gradient increased to >40 mmHg (11/62, 17.4%) in 11 patients during follow-up, leading to surgical explantation in two, balloon dilatation in five, and a Melody-in-Melody procedure in four (Online Table 2). Univariate analysis identified no factors that were significantly associated with the need for treatment of restenosis. Excluding deaths and explanted valves but including interventional treatments, mean RVOT pressure gradient was significantly reduced six months after the initial PPVI (p<0.001) and was sustained at all follow-up time points (Figure 3A). Linear regression of mean RVOT gradient at six months and five years after implant showed no impact of any of the factors analysed.
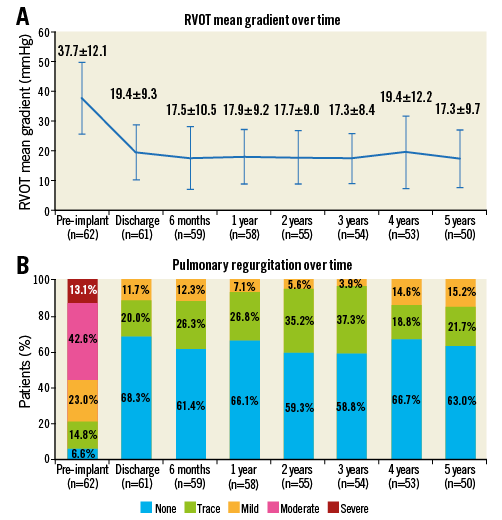
Figure 3. Echocardiographic data. A) Mean RVOT gradient over time. B) PR classification over time.
Before PPVI, 55.7% of patients had moderate/severe PR on echocardiography, but at discharge none did (p<0.001). This result was sustained at five years (Figure 3B) except in one patient with iatrogenic valve regurgitation after balloon dilatation of a valve stenosis post endocarditis. This patient required a Melody-in-Melody procedure for significant PR.
Figure 4 illustrates freedom from PPV dysfunction, freedom from RVOT conduit reoperation, and freedom from catheter reintervention. A log-rank test identified no significant factors associated with PPV dysfunction. A Melody-in-Melody reintervention was performed in eight patients, due to PPVI stent fracture in six patients and stenosis with gradient >40 mmHg in four patients (both in two patients).
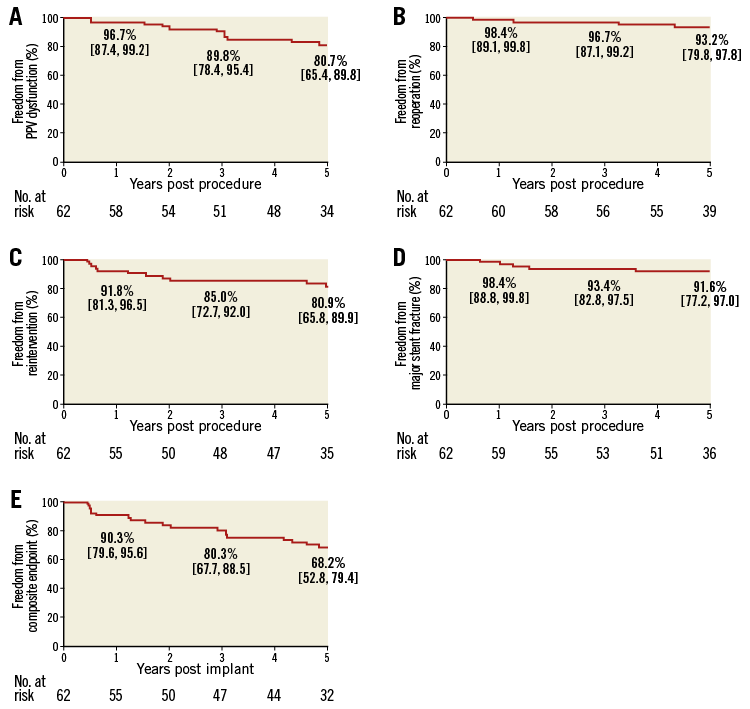
Figure 4. Kaplan-Meier curves to five years post PPVI. A) Freedom from PPV dysfunction. B) Reoperation. C) Reintervention. D) Major stent fracture. E) Composite endpoint. The numbers in the plots represent the Kaplan-Meier estimate and the 95% confidence interval.
SERIOUS ADVERSE EVENTS
Eighteen patients (28.6%) experienced 26 device-related SAE, including valve dysfunction/stenosis (11), major stent fracture (five), subacute bacterial endocarditis (four), coronary compression causing myocardial ischaemia (one), valve dysfunction/regurgitation (one), other cardiac events (four), and other device-related events (two). The five-year freedom from major stent fracture estimate was 91.6% (Figure 4D). Additional details are provided in Online Table 2.
TOTAL CLINICAL PERFORMANCE
The primary composite endpoint of the study is presented in Figure 4E. At five years after implant, freedom from this composite endpoint was 68.2%, with 32 patients displaying good clinical performance and another 11 treated with either balloon dilatation or a Melody-in-Melody valve procedure resulting in a desirable clinical outcome.
QUALITY OF LIFE
In the 44 patients who completed the QOL questionnaire at baseline, the mean utility index was 0.818±0.137 at baseline, 0.795±0.201 at six months (n=41), and 0.890±0.189 at five years (n=35). The VAS improved from 72.3±20.2 at baseline to 83.1±12.2 at six months to 85.2±14.4 at five years (Online Table 3). The utility index was significantly improved at both six months and five years after implantation, as was the VAS at the same time points (Figure 5).
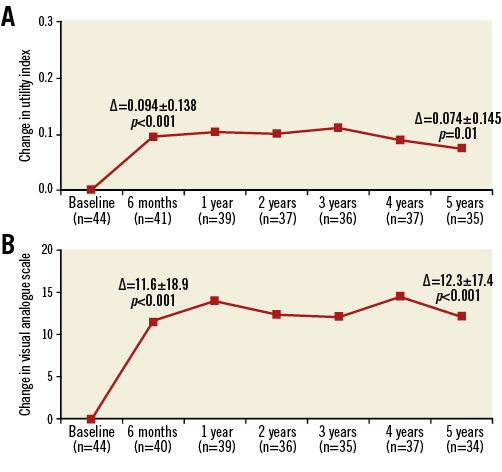
Figure 5. EQ-5D utility index and VAS changes from baseline over time. A) EQ-5D index. B) VAS. Data are from all subjects with paired data. p-value from Wilcoxon signed-rank test.
Many patients self-reported their baseline QOL as good (Online Table 3). Thus, the majority of patients reported no change in status at six months and five years. Among patients who reported a change, the majority reported improvements in QOL outcomes.
To assess the impact of mean RVOT gradient improvement on change in the EQ-5D utility index, a linear regression was performed for the two variables from baseline to six months and from baseline to five years. There was no significant relationship from baseline to six months (slope=-0.0002, p=0.88) or from baseline to five years (slope=-0.0030, p=0.11). However, when the same linear regression was performed comparing the VAS and mean RVOT gradient improvement, there was a significant relationship from baseline to five years (slope=-0.625, p=0.001) but not from baseline to six months (slope=-0.212, p=0.33).
No significant impact of improved PR on QOL improvement was identified for either baseline to six months (VAS, slope=6.84, p=0.46; EQ-5D index, slope=0.066, p=0.32) or baseline to five years (VAS, slope=6.13, p=0.35; EQ-5D index, slope=-0.04, p=0.56).
Discussion
This study showed that PPVI persistently reduced clinically significant PR and RVOT stenosis and led to a long-lasting improvement in QOL in patients with initial dysfunction of the RVOT conduit up to five years.
This study confirmed the high procedural success rate of PPVI (>90%)2, but also reiterates the procedural mortality related to PPVI. The main reasons reported are coronary artery compression by the implanted valve or by calcified material from the conduit that is dislocated during PPVI2. The current practice of performing coronary angiography simultaneously with inflation of a high-pressure balloon in the RVOT may reduce the risk of coronary compression. Rupture of a calcified conduit is also potentially fatal and treated only by rapid implantation of a covered stent or by surgery. It is speculative whether the use of covered pre-stents in the calcified stenotic RVOT will reduce the risk. Other complications, such as valve embolisation4 or pulmonary artery bleeding caused by an injured vessel wall from the stiff guidewire, may also be prevented. To minimise mortality, PPVI should only be performed in experienced hands and in centres where the complications can be appropriately handled.
Haemodynamics after PPVI are excellent in restoring pulmonary competence. Even the first reported Melody valve implantation successfully reduced PR1. This was confirmed in other institutional reports5,17,18, in registry studies4 and in prospective cohort studies2, where PR could be removed completely or reduced to trivial, even in patients with severe pulmonary hypertension19. Reports of patients with moderate or severe regurgitation are mainly found after an episode of endocarditis20. Our study shows that the re-established pulmonary valve competence can be sustained for at least five years. This is in contrast to surgically implanted bovine jugular vein valves, which are reported to become regurgitant in 7-15% of patients at early and midterm follow-up21,22. It is speculated that fixation of the valve in the stent not only improves adaptation of the leaflets on early follow-up, but probably also prevents mechanically induced degeneration in the longer term.
Our study also confirms that there is prolonged significant reduction of RVOT stenosis, although a number of patients with residual stenosis must undergo reintervention in the first few years after implantation. Stenosis was the most common indication for surgical or catheter reintervention. However, absolute numbers were low. The Kaplan-Meier estimate for five-year survival without reintervention was similar to the 71% freedom from reintervention reported for surgical bovine jugular vein valves22. Currently, the recommendation is to place one or more pre-stents to eliminate any residual stenosis and stent fracture and to reduce conduit recoil during balloon deflation before PPVI15. This practice has reduced the incidence of stent fractures, residual stenosis, and reinterventions11. As such, these data may overestimate the rate of restenosis and reintervention if current recommendations are applied properly.
Endocarditis after PPVI is a serious event. This study identified four cases of bacterial endocarditis. Three were included in a previous analysis focusing on endocarditis after Melody implantation and discussed in detail there23.
Favourable haemodynamics result in persistently improved functional class11. However, NYHA functional classification is based on anamnestic data and the physician’s subjective impressions. Self-reported short-term health-related QOL (SF-36) was excellent with improved perception of physical function and general health13. In this study we confirmed early and significant improvements with a more generalised instrument (EQ-5D).
We could not define any subgroups that had more or less improvement in QOL. Our data concerning a difference in outcomes, based on whether PPVI was performed to eliminate pure stenosis or regurgitation, or a combination, were also inconsistent, whether the EQ-5D VAS or the EQ-5D index was used. This is not in agreement with the early London data on exercise capacity, where improvements could be shown in patients with RVOT obstruction treated with PPVI but not in those with PR6,7. As speculated13, this might be due to the restrictive PPVI indication in the current study, which allows PPVI for severe regurgitation only in symptomatic patients or patients with RV dilatation or dysfunction.
Many patients felt that their health-related QOL was rather good before PPVI. However, both the detailed questions about health status and the self-scoring by the VAS identified an immediate significant improvement after the implant. The current study extends the data on patient-reported outcome to persistently improved QOL for at least five years. The gain in health-related QOL can be expected to translate into a meaningful gain in quality-adjusted life years when considering an untreated cohort that, conservatively, could be assumed to maintain the baseline QOL. However, our study does not allow a proper cost-utility ratio, as no data for the alternative treatment, surgery, are assessed, and the costs for both PPVI and surgery vary tremendously from country to country.
Limitations
Due to the small number of procedural failures in this study, a regression analysis to identify predictors of procedural success yielded no significant findings. Furthermore, the analysis of other factors that might have had an impact on the haemodynamic results or QOL may have suffered from the low number of patients. Also, the improvements in QOL neglect patients who died or underwent surgery during follow-up.
Conclusions
This study reveals that PPVI resolves clinically significant PR and reduces RVOT stenosis in patients with initial dysfunction of the RVOT conduit up to five years after the procedure. It leads to a persistently improved QOL. However, complications are described, which might be preventable in experienced hands, or at least manageable in appropriately equipped centres.
| Impact on daily practice In experienced hands PPVI is an alternative to surgery for the treatment of a dysfunctional RVOT conduit. |
Acknowledgements
We thank Jessica Dries-Devlin, PhD, and Colleen Gilbert, PharmD, for editorial support and assistance with drafting the methods, results, and figures. We also thank Minglei Liu, PhD, for statistical analysis support, and Marieke Gerards, Kristin Boulware, Marta Mourinho, Mandi Rupp, and Patrick Simons for overall study management support. All are Medtronic employees.
Funding
Medtronic sponsored and funded this study.
Conflict of interest statement
A. Hager reports grants and personal fees from Medtronic. S. Schubert reports grants from Medtronic and Novartis and personal fees from Medtronic and Gore. P. Ewert reports personal fees (proctor) for the Medtronic Melody valve and is a proctor for the Edwards pulmonic transcatheter valve. L. Søndergaard is a proctor for Medtronic. M. Witsenburg is a proctor for Medtronic. L. Benson is a Melody valve proctor for Medtronic. J.S. de Lezo has received speaking fees from Medtronic. T. Lung is an employee of Medtronic. A. Eiken and J. Hess are proctors for the Medtronic Melody valve. F. Berger and P. Guccione have no conflicts of interest to declare.
SUPPLEMENTARY DATA
Appendix
SUPPLEMENTARY METHODS
INCLUSION/EXCLUSION CRITERIA
Inclusion criteria were age five years or older, weight of 30 kg or greater, existence of a full circumferential RVOT conduit 16 mm or greater in diameter when originally implanted, and either moderate/severe pulmonary regurgitation (PR) or mean RVOT pressure gradient 35 mmHg or greater for patients in New York Heart Association (NYHA) Class II or greater. Patients in NYHA Class I were required to have severe pulmonary regurgitation with right ventricular (RV) dilatation or dysfunction, or a mean RVOT pressure gradient greater than or equal to 40 mmHg.
Exclusion criteria included active endocarditis, major or progressive non-cardiac disease, obstruction of the central veins, known intravenous drug abuse, or patients who did not give legal consent or did not want to comply with follow-up requirements.
QUALITY OF LIFE ASSESSMENT
The EQ-5D questionnaire provides a standardised measure of health outcome through a descriptive profile of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The utility index is reported on a scale from 0 to 1, while the VAS is reported on a scale of 0 to 100. Values here are reported as the mean change from baseline±standard deviation. Data were as available from 44 patients >15 years old; there were two patients who only had discharge or pre-implant EQ-5D data available with no further follow-up time points, so these patients were excluded from the analysis on paired data. A sensitivity analysis was performed to evaluate the impact of these missing data.
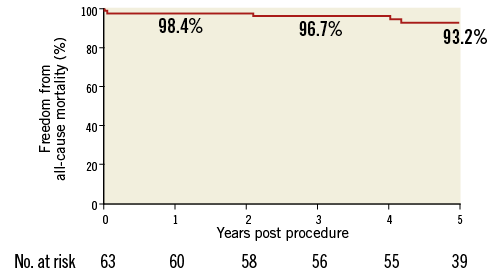
Online Figure 1. Freedom from all-cause mortality.

