
Abstract
Aims: The aim of this study was to assess the international procedural and short-term to midterm experience with the new percutaneous Venus P-valve.
Methods and results: Retrospective data of patient characteristics, clinical and imaging follow-up of Venus P-valve implantation outside China were collected. Thirty-eight patients underwent attempted Venus P-valve implantation between October 2013 and April 2017. Thirty-seven valves were successfully implanted during 38 procedures. There was one unsuccessful attempt and there were two valve migrations, one of which required surgical repositioning. The mean follow-up was 25 months with no short-term or midterm valve failure or deterioration in performance. Frame fractures occurred in 27% of patients. The cohort demonstrated a statistically significant reduction in pulmonary regurgitation fraction and indexed right ventricular diastolic volumes at six and 12 months.
Conclusions: Implantation of the Venus P-valve has provided satisfactory short-term to midterm results with high success and low complication rates in an inherently challenging patient substrate.
Abbreviations
CHD: congenital heart disease
CXR: chest X-ray
ECG: electrocardiogram
LPA: left pulmonary artery
MHRA: Medicines and Healthcare Products Regulatory Agency
MPA: main pulmonary artery
MRI: magnetic resonance imaging
PA: pulmonary artery
PPVI: percutaneous pulmonary valve implantation
RPA: right pulmonary artery
RVOT: right ventricular outflow tract
TTE: transthoracic echocardiography
Introduction
Percutaneous pulmonary valve implantation (PPVI) was initially limited to patients who had circumferential surgical right ventricular outflow tracts (RVOT), for example conduits or bioprosthetic valves, but its efficacy is now being demonstrated in patients who have had RVOT reconstructions with a transannular patch1,2. RVOT size remains a critical determinant of suitability for PPVI.
The Venus P-valve™ (Venus Medtech, Shanghai, China) is a new, percutaneous pulmonary valve designed specifically for the native RVOT. Its self-expanding design can be accommodated in RVOT diameters up to 32-33 millimetres, which is larger than commercially available valves. Early experience has been encouraging, suggesting that the Venus P-valve can be implanted safely, with good short-term results in patients with native RVOT3-6.
The aim of this paper was to report retrospectively collected procedural and early to midterm follow-up data of flared Venus P-valves implanted on compassionate use grounds. Data were gathered from an international multicentre collaborative group excluding China, where the valve has undergone a separate regulatory study. Data from seven patients in two centres included in this manuscript have been included in previous publications4,5.
Methods
STUDY DESIGN
This was a multicentre, international, retrospective study of compassionate use cases of the Venus P-valve. The study was approved by the ethics committee of each hospital. The procedural indications as well as the probable suitability of each patient’s outflow tract anatomy were reviewed by a team led by the senior author of this manuscript before implantation. Eleven institutions (listed in the authors’ affiliations) in six countries participated. The authors agreed that a technically successful valve implantation would be defined by the presence of a functioning Venus P-valve in the RVOT 24 hours after the procedure. Major adverse events were defined as death, unplanned surgery or additional consequential intervention, new-onset moderate or severe tricuspid regurgitation, bacterial endocarditis, cardiac arrest or new clinically significant ventricular arrhythmia during the course of follow-up.
POPULATION
Data were available on patients brought to the catheter laboratory between May 2013 and April 2017 in all 11 centres outside China which had obtained compassionate use permission from their local responsible regulatory bodies for Venus P-valve implantation. As well as meeting local guidelines for pulmonary valve replacement, patients were considered for the Venus P-valve if the Melody® (Medtronic, Minneapolis, MN, USA) or SAPIEN™ (Edwards Lifesciences, Irvine, CA, USA) valve was not approved in that country, or if the patient’s anatomy was not suitable for these valves because of the size of the RVOT. All patients had significant pulmonary regurgitation (PR) following previous surgery (PR grade ≥3+ by transthoracic echocardiography [TTE] using colour flow Doppler echocardiography). Patients also either had symptoms attributable to their PR, or fulfilled the following cardiovascular magnetic resonance imaging (CMR) criteria: indexed RV end-diastolic volume (RVEDVi) exceeding 150 ml/m2, or RV ejection fraction (EF) ≤45% or PR fraction (PRF) ≥30%. Data from CMR studies were also used to assess the size and the morphology of the RVOT, and the main pulmonary artery (MPA) and its branches qualitatively and quantitatively7.
Patients were not brought to the cath lab for Venus P-valve implantation if they had significant obstruction of the pulmonary artery branches likely to require an additional intervention, obstruction of the central veins preventing delivery of the Venus P-valve system, or cross-sectional imaging evidence of an outflow tract and coronary artery relationship which may have risked coronary artery compression.
Data were collected in 38 patients brought to the catheter laboratory with the intention of implanting a flared Venus P-valve (Table 1). Their ages ranged from 11 to 76 years (mean 24±14 SD), and their weights from 28 to 98.5 kg (mean 59±16.2 SD). Thirty-six of the 38 patients had undergone previous repair of tetralogy of Fallot or pulmonary atresia with ventricular septal defect (VSD) with a transannular patch. The other two patients had RVOT dysfunction secondary to a Ross procedure and secondary to repair of truncus arteriosus. In one patient, a technical failure of the valve delivery system during deployment necessitated urgent surgical pulmonary valve replacement.
Preprocedural CMR, available in 36 patients, showed a mean RVEDVi of 151 ml/m2 (±SD 24 ml/m2); mean RVEF of 48% (±SD 8%), and mean PRF of 48% (±SD 12%). In 17 patients, the RVEDVi was less than 150 ml/m2, with five of those being less than 130 ml/m2. These 17 patients were symptomatic and 16 of 17 had a PRF ≥30%, with five having an RVEF ≤45%.
PATIENT EVALUATION
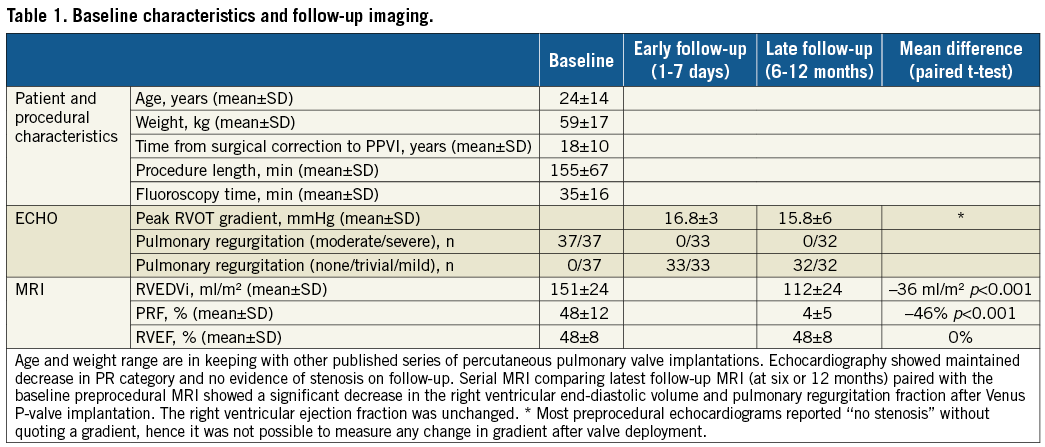
All patients underwent diagnostic cardiac catheterisation as part of the assessment of suitability. Biplane angiography was performed to assess the main pulmonary artery (MPA) diameters at three levels (Figure 1): distal MPA diameter just proximal to the bifurcation, mid MPA, and the RVOT/MPA junction. All measurements were taken at maximum systolic expansion. RV angiography was performed with a pigtail catheter in the RV during occlusion of the RVOT by a fully inflated AMPLATZER™ ASD (St. Jude Medical, St. Paul, MN, USA) sizing balloon. Multiple measurements of the fully expanded balloon were taken to define constrictions as well as the maximum expansion of the balloon. Finally, with the balloon fully inflated in the PA, selective coronary angiography was performed to assess the risk of coronary artery compression during valve implantation8.
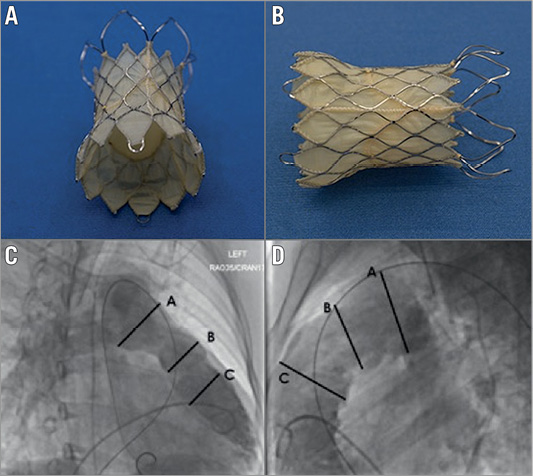
Figure 1. The Venus P-valve. The nitinol frame here is fully expanded showing the covered RVOT end and the bare metal pulmonary bifurcation end. The attachment “ears” can be seen extending beyond the true valve frame (A & B). Measurements of the RVOT MPA, taken at three levels in two projections (C & D). These aim to define the diameters at the distal MPA just before the bifurcation, the mid MPA, and the proximal RVOT/MPA, marked A, B and C, respectively. All the measurements were taken at maximum systolic diameters.
As well as confirming suitability, balloon testing provided the team with information to determine the likely valve size required for implantation.
VALVE AND DELIVERY SYSTEM
The Venus P-valve is a trileaflet porcine pericardial tissue valve. The pericardium is sutured onto the self-expanding nitinol stent at multiple levels in the mid part of the stent (Figure 1). The diameters and lengths of the middle part of the flared valve range from 22 to 36 mm, and 20 to 35 mm, respectively. The proximal and distal flares generate an hourglass configuration which helps to stabilise the valve in the RVOT and main pulmonary artery. The mid portion and proximal stent flare are covered by porcine pericardial tissue. There are two attachment “ears” on the proximal flare, which facilitate loading of the valve, and radiopaque markers at either end of the middle segment of the stent.
The delivery system consists of a distal capsule, which contains the loaded valve and is 19-24 Fr (depending on the valve size), and a 15 Fr 100 cm-long shaft. The loading procedure involves crimping the valve with a proprietary crimping device after submerging it in a sterile cold saline solution, which facilitates crimping by decreasing the memory properties of the nitinol.
VALVE SELECTION
Valve diameter is determined by measuring the balloon waist and then oversizing by 2-4 mm, and by considering the maximum angiographic systolic diameter of the mid and distal MPA to ensure apposition of the valve to the wall of the MPA. With differences in the measured diameter of 1-2 mm affecting the chosen valve size, balloon interrogation can reveal diameter differences of a magnitude that materially affects the size of the valve chosen (Figure 2). The chosen length of the valve should be similar to the length from the RVOT from the estimated valve annulus position to the PA bifurcation.
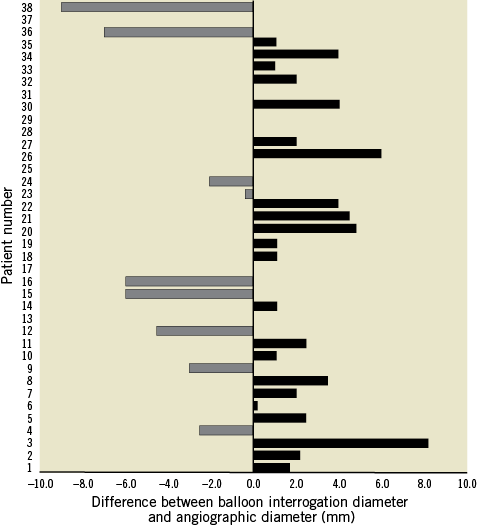
Figure 2. Angiographic MPA measurement and compliant balloon measurements. Differences of nearly 10 mm were seen in some cases (mean difference=3 mm). Although in several cases balloon testing yielded a smaller diameter than the angiogram (9/38), in the majority of cases (23/38) balloon assessment measured larger than angiography. These discrepancies are explainable by the compliance of a native outflow tract, the degree of dynamic alteration in diameter and morphology that accompanies severe regurgitation, and often the complexity of the chronically dilated RV.
PROCEDURE
VALVE IMPLANTATION
Valve deployment was performed under general anaesthesia from the femoral vein in all except one patient, in whom the right internal jugular vein was used because of interruption of the inferior vena cava (IVC)5. The valve was advanced through the right side of the heart over a distally placed Lunderquist® guidewire (Cook Medical, Bloomington, IN, USA) and into a proximal branch pulmonary artery. Frequent check angiograms were performed during positioning and deployment of the valve. The distal flare was exposed in the proximal left pulmonary artery (LPA). The rest of the valve was then deployed slowly, whilst carefully withdrawing the system to allow the distal flare to slide out of the LPA origin into the MPA (Figure 3). Angiography and repeat haemodynamics were performed to check the position and competence of the valve.
Figure 3. Deployment of the flared Venus P-valve. These panels show the importance of careful, staged positioning of the Venus valve. A) The deployment starting position in the proximal LPA. After exposing the distal flare, the system is pulled free from the LPA origin (B & C) before gradually releasing the rest of the valve frame (D), using intermittent low-volume angiography from a pigtail catheter placed in the RV for fine adjustments.
Antibiotics were given according to the hospital protocols. The patients were discharged on oral aspirin at 3-5 mg/kg/day for life with or without clopidogrel for three to six months.
FOLLOW-UP
The patients were assessed one day after the procedure and at one, three and six months, then yearly thereafter. The recommended follow-up evaluation included clinical assessment, electrocardiogram (ECG), chest X-ray (CXR) and TTE. Fluoroscopy was recommended at three to six months to check for frame fractures. CMR was recommended at six-month and one-year follow-up to assess the function of the implanted valve, and RV dimensions and function.
STATISTICAL ANALYSIS
Continuous variables are expressed as mean+standard deviation (SD) or median (interquartile range [IQR]), while categorical variables are presented as a number. A comparison of the parameters before and after the pulmonary valve implantation was analysed using Wilcoxon signed-rank tests. A p-value of less than 0.05 was considered to be statistically significant.
Results
The Venus P-valve was implanted successfully in 37 of 38 patients. The diameter of the 37 successfully implanted Venus P-valves ranged from 24 to 36 mm, with a length of 30 mm in 32 patients, and 35 mm in 5 patients. The mean procedure time for the whole population was 155±67 minutes (range 45-312 minutes). The mean fluoroscopy time was 35±16 minutes (range 13-89 minutes).
PROCEDURE-RELATED COMPLICATIONS
Implantation was not completed in one patient because of a break in the sheath covering the valve during manipulation across the RVOT. The patient had undergone prior LPA stenting; this caused difficulty in advancing into the LPA, resulting in damage to the delivery system. The valve frame protruded from the split in the sheath and therefore, to avoid intracardiac damage, the valve assembly was removed surgically with concomitant, uncomplicated surgical pulmonary valve replacement. One other patient had valve deployment in the presence of a previously implanted LPA stent. Although, on retrospective discussion, positioning the delivery system was subjectively challenging in that case, there was no complication. Following the experience with the broken sheath, prior LPA stenting has been considered as a relative contraindication for Venus P-valve implantation.
There were three other procedure-related complications. Two of these involved valve migration. In one patient, incomplete detachment of the delivery system led to the valve being pulled slightly proximal into the RV. This caused interference with the tricuspid valve, increasing the patient’s tricuspid regurgitation from mild to moderate. Fluoroscopically and angiographically the valve position still looked appropriate and it was functioning correctly; therefore, it was left in situ. The tricuspid regurgitation remained moderate and, despite this, the patient’s RV has shown signs of positive remodelling and eventual decrease in size. In another patient, valve migration was due to undersizing of the valve in relation to the RVOT. The valve position was considered to be unstable, hence the patient was brought urgently to the operating room where the Venus P-valve was repositioned and sutured in place. This valve was functioning well at follow-up with no evidence of dysfunction or paravalvar leak. In one patient, with pre-existing hypoplasia of the right pulmonary artery (RPA), the origin of the RPA was obstructed by the valve frame with minimal angiographic flow. Following repositioning of the valve, by grasping the attachment ear on the proximal extent of the frame with a bioptome, an RPA stent was successfully placed with a satisfactory haemodynamic and angiographic result (Figure 4).
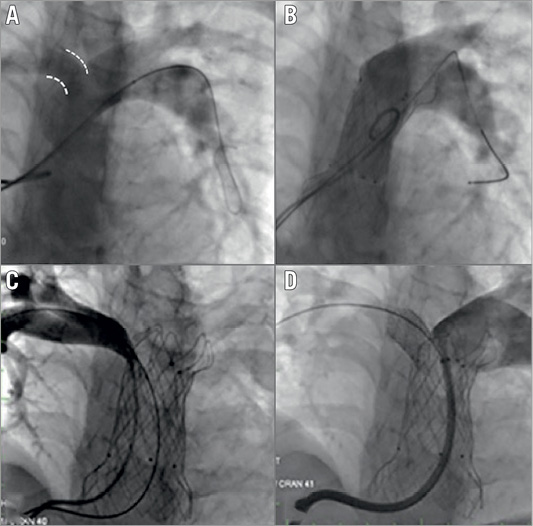
Figure 4. RPA occlusion during valve deployment. A) Discrepancy in the RPA and LPA sizes in this 39 kg 12-year-old patient. After valve deployment (B) there is no flow into the RPA. After grasping the proximal (ventricular) flare with a bioptome, the valve was pulled back slightly, resulting in positive reorientation and some flow into the RPA. As the origin of the RPA still looked partially compromised (C), we placed a balloon-expandable stent in the RPA origin with good angiographic effect (D).
Follow-up
The mean follow-up was 25 (±8) months. One patient was lost to follow-up after six months. Follow-up data at a one-year time point are available in 15 of the 37 implanted patients. Nine patients had fever for one to two days immediately after the procedure without other signs or laboratory markers of infection. This may have been due to inadequate saline rinsing of the valve. Early on in the experience, seven of 13 patients had post-procedural fever. Subsequently, the valve was rinsed in three bowls of normal saline for five minutes each. This significantly reduced the incidence of fever to two out of 24 subsequent patients. One patient had an infected haematoma at the groin puncture site which resolved with antibiotic treatment. There has been no reported clinical, imaging or laboratory evidence of endocarditis.
One patient had transient premature ventricular contractions (PVCs) for two days after the procedure, which settled spontaneously.
TTE evaluation one day after the procedure revealed a well-functioning pulmonary valve with no paravalvar leak or RVOT obstruction in all the patients. All previously symptomatic patients reported subjective improvement in their symptoms during the follow-up. Eight patients have so far completed their three-year follow-up with echocardiography showing no evidence of valve stenosis or regurgitation. Thirty-five patients had either no evidence of PR or trivial PR throughout follow-up. One patient had mild PR immediately after valve deployment and continued to have mild PR on follow-up. One patient has been noted to have a mild paravalvar leak during follow-up. No patient seen at follow-up at six or 12 months has demonstrated significant or progressive RVOT obstruction or pulmonary regurgitation.
Follow-up fluoroscopy was performed in 30 patients at a mean of 9.6 months (range 1-12 months) after valve implantation. Frame fractures were noted in eight (27%) patients, all in the proximal flare of the stent. In seven cases, single wire fractures were noted at three-month follow-up. Two of these were on the delivery attachment ear, not in the true structure of the stent. One patient had three individual wire fractures noted at one-year follow-up (Figure 5). Serial fluoroscopy performed in all these cases has not shown evidence of further fractures. On echocardiography, the valve function has remained unaffected. CT scans have not been performed.
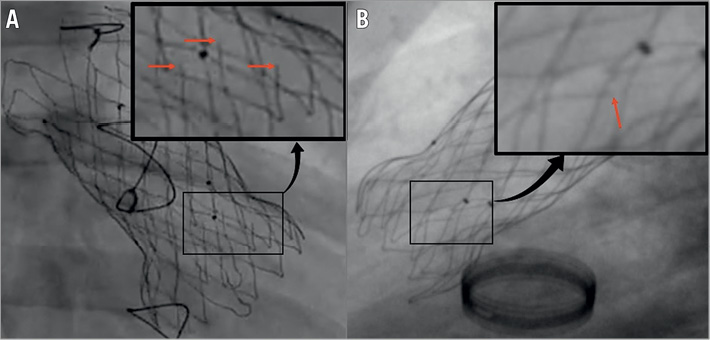
Figure 5. Frame fractures. Two examples of fractures in the ventricular flare of the valve frame. A) Three frame fractures, noted at one-year follow-up. B) A single wire fracture noted at three months. Neither case showed any signs of dysfunction of the implanted valve. Fractures are highlighted in the magnified box by red arrows.
Follow-up CMR data (Table 1) were available for 23 of 37 patients, 22 at six months and 15 at 12 months. At the latest MRI follow-up, the overall mean PRF had reduced from 48% before to 4% after the valve implantation. The RVEDVi decreased from a mean of 151 ml/m2 before to 112 ml/m2 after valve implantation. The patient who had tricuspid regurgitation following proximal valve migration interfering with the tricuspid valve saw an initial increase in his RVEDVi from 147 ml/m2 to 174 ml/m2 at six-month follow-up, which subsequently decreased to 120 ml/m2 on CMR 12 months after valve implantation.
Discussion
The Venus P-valve has been developed to address the challenges posed by the dilated native outflow tract, making it possible to implant valves in a wider range of patients, especially those patients who have previously had a surgical transannular patch repair of tetralogy of Fallot.
As with other valves, critical evaluation of the outflow tract characteristics is the key to the selection of patients and successful planning and performance of Venus P-valve implantation. The size of the RVOT, as measured by CMR, differs from the fluoroscopic balloon interrogation diameter which, in our experience, is the most reliable for the selection of the valve diameter. Although CMR is an important initial investigation in order to identify a potentially suitable anatomy and provide data which are influential in determining indications for intervention, in this cohort of patients with dilated outflows, final decisions cannot be made without invasive balloon interrogation of the outflow tract9. Experience has shown us that the prior placement of a stent in the branch PAs can cause significant difficulty in advancing the Venus P-valve delivery assembly into the stent lumen, hindering the ability to deploy the valve safely and effectively. Prior stenting of a branch PA should be a deterrent if not a contraindication to Venus P-valve implantation.
SUCCESS RATE OF VALVE IMPLANTATION
Technical success in this series was high with successful valve implantation in 37/38 (97%) attempts, albeit that one of these 37 patients required surgical stabilisation of the percutaneously deployed valve. In US clinical trials, Melody valve implantation success was similarly high at 97% (29 out of 30 attempts)10. SAPIEN valve implantation was achieved in 33 out of 34 attempts (97%)11. Venus P-valve implantation compares well with the Melody and SAPIEN valves, particularly considering the challenging anatomical substrates in which Venus P-valve implantation may be attempted.
The length and diameter of the valve significantly affect the stiffness of the loaded delivery system. This is particularly relevant when faced with challenging anatomy such as stenosis, unusual angulation or pre-existing pulmonary artery stents. Difficulty in passing the delivery system into the LPA is probably multifactorial, relating to the interaction between the anatomy and the relative stiffness of the delivery system after loading the valve, exaggerated by the presence of a stent in a branch PA, factors which were relevant in the case where implantation failed.
VALVE MIGRATION AND EMBOLISATION
Valve migration and embolisation remain a concern for PPVI, particularly in the absence of a previous conduit in the RVOT. Our study showed a 5% incidence (n=2) of valve migration. There were no gross valve embolisations, probably as the design of the valve stent will limit the ability of the valve to change its position, even if undersized.
However, migration of only a few millimetres had serious consequences for both of our patients, requiring cardiac surgery to stabilise its position in one case and causing significant interference with the tricuspid valve in the other. The literature suggests a higher risk of embolisation with the SAPIEN valve, with three of 33 (10%) of the initial cohort of the SAPIEN valves embolising. Two of these patients required surgical retrieval10-12.
FRAME FRACTURE
Fracture rates with the Melody valve have been reported as being 21.1% by Nordmeyer et al13 and, in the US Melody valve trial, stent fracture was seen in 23% at 14 months and 40% at 30 months10. There are no published reports of stent fracture of the SAPIEN valve in the pulmonary position.
In our study, stent fractures have been sought on fluoroscopy rather than CXRs to improve detection. Frame-by-frame fluoroscopic analysis identified that eight out of 30 (27%) of the valve frames had wire fractures during follow-up. Using just CXR, this incidence dropped to 22%. However, we do not feel that CXR is sensitive enough, hence our higher reported incidence. This is higher than the incidence in Melody valves during a comparable length of follow-up.
All the stent fractures were in the region of the proximal flare. The fractures have not affected the function of the valve or frame integrity or stability, even in the patient with three wire fractures. As the proximal flare is positioned in the muscular RVOT, it is not surprising that wire fractures occurred there, most likely as a result of repetitive muscular contraction of the RVOT.
INFECTIVE ENDOCARDITIS
Endocarditis is an important issue after PPVI, with an annualised incidence of 2.4%14,15. It may be that the anatomical substrate into which the valve is implanted is as important as the valve itself, with the environment of a stenosed, ragged conduit perhaps being more conducive to infection of a newly implanted percutaneous valve than the dilated, minimally prosthetic environment offered by a transannular patch and into which we place the Venus P-valve. The absence of endocarditis at a mean follow-up duration of 25 months should not provide false reassurance that endocarditis is not a risk for the Venus P-valve. Continued follow-up evaluation is essential to avoid false reassurance and identify the true incidence of endocarditis with the Venus P-valve.
Limitations
This study suffers from the usual flaws of a multicentre, retrospective cohort. Without a prospective protocol, it is not known how many patients were screened in and out of contention based on data reviewed by the patient’s primary cardiologist. In compassionate use cases, closer clinical, and clinical investigative follow-up plans are usually suggested. All data were self-reported and, although the preprocedural imaging studies were prospectively assessed by the senior author to determine suitability, this was carried out without a prospective protocol. There was no true core lab, and the results of the post-procedural investigations were reported by the clinicians locally involved.
Conclusions
This study shows satisfactory procedural as well as short-term and midterm outcomes for the Venus P-valve. These are encouraging results, spanning the learning curve for indications and practical use of this valve which was designed for an anatomically challenging group of patients. The P-Valve may provide an important percutaneous option for patients with larger outflow tracts following surgical repair and expands the applicability of PPVI beyond the Melody and SAPIEN valves in RVOTs up to 33 mm diameter. Although the early results are satisfactory, meticulous long-term follow-up is required.
| Impact on daily practice This international experience with the Venus P-valve suggests that it provides a viable alternative to surgical reconstruction in patients with dilated outflow tracts and severe pulmonary regurgitation. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

