
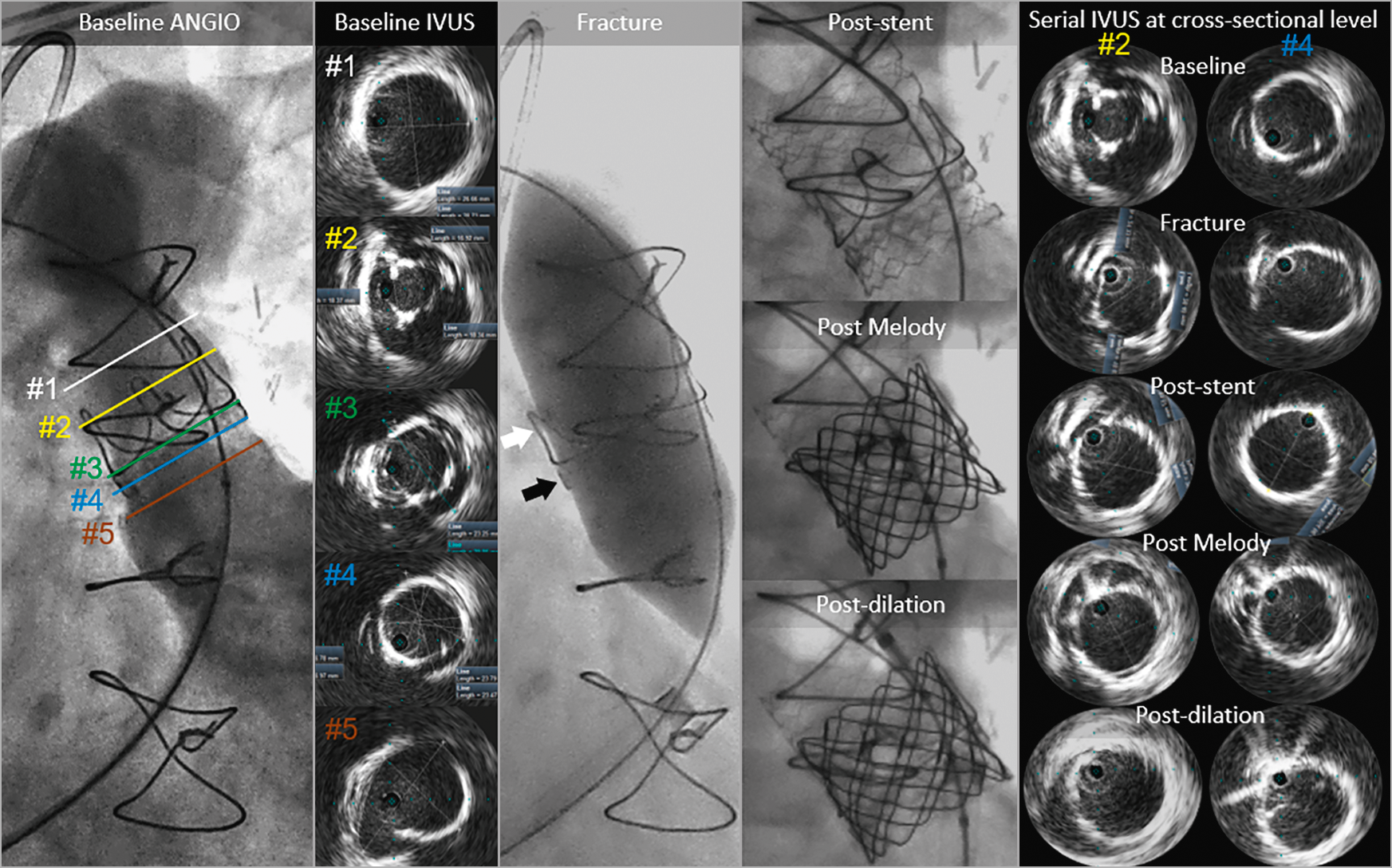
Figure 1. Corresponding angiography and IVUS images of the RVOT anatomy, obtained sequentially during the intentional fracture of a 23 mm diameter Magna bioprosthesis (using an Atlas GOLD 24 mm at 18 atm) prior to valve-in-valve transcatheter pulmonary valve replacement with a 22 mm diameter Melody pulmonary valve.
A 28-year-old male with tetralogy of Fallot underwent total correction at the age of five with a transannular patch of the pulmonary valve. Fifteen years later, he had a 23 mm diameter Magna bioprosthesis (Edwards Lifesciences, Irvine, CA, USA) inserted at the pulmonary position due to pulmonary valve insufficiency. He was scheduled for valve-in-valve transcatheter pulmonary valve replacement (TPVR) due to symptomatic bioprosthesis dysfunction (insufficiency and stenosis).
Intravascular ultrasound (IVUS) (Visions PV .035 Digital Catheter; Philips Volcano, San Diego, CA, USA) visualised right ventricle outflow tract (RVOT) cross-sections (Baseline IVUS corresponding to Baseline ANGIO #1-5 in Figure 1) revealing immobile cusps with a narrowed valve orifice (86.2 mm2, #2) and permitting an excellent insight into the structure of the bioprosthesis posts (#2) and ring (#3-4). RVOT dimensions above the bioprosthesis stent posts were 26.7×28.7 mm (#1), with inner ring diameters of 19.4×20.2 mm (#4) and outer ring diameters of 23.4×23.7 mm. IVUS also visualised the 26.8×27 mm native annulus outside of the ring (#4). RVOT, just beneath the valve ring, was elliptical with an area of 635.5 mm2 (derived diameter of 28.4 mm) (#5).
The Magna frame was fractured using an Atlas® GOLD 24 mm at 18 atm (Bard Peripheral Vascular, Tempe, AZ, USA), with a subsequent increase in valve frame dimensions (Fracture panel; black arrow indicates the fracture site) and bioprosthesis post motion (Fracture panel; white arrow indicates the stent’s post) to 22.8×24.5 mm (399.5 mm2, Serial IVUS panel, column #4). A 36 mm bare metal stent (IntraStent™ LD Max; ev3, Plymouth, MN, USA) was deployed on a 24 mm balloon-in-balloon catheter (BIB®; NuMED, Hopkinton, NY, USA, Post-stent panel). IVUS verified stent expansion, oval shape, and stent inner diameters of 21.8×22.5 mm (354.7 mm2, Serial IVUS panel, column #4). A Melody™ pulmonary valve (Medtronic, Minneapolis, MN, USA) was deployed on a 22 mm Ensemble® transcatheter delivery system (Medtronic) (Post Melody panel). IVUS verified inner valve diameters of 20.9×21.3 mm (348.0 mm2, Serial IVUS panel, column #4). After post-dilation with an Atlas GOLD 24 mm at 16 atm, IVUS inner valve frame dimensions increased to 21.8×23.1 mm (358.7 mm2) with symmetrical leaflet motion (Serial IVUS panel, column #4). There was no RVOT gradient.
Conflict of interest statement
G.S. Mintz has received honoraria from Boston Scientific, Philips, Medtronic, and Terumo (but none allied to the current manuscript). M. Demkow is proctoring for Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

