
Abstract
Aims: Our aim was to report the first five cases of percutaneous implantation of the Venus P-valve® in Europe.
Methods and results: We successfully implanted Venus P-valves in five consecutive cases in patients whose anatomy was thought to be unfavourable for currently available percutaneous valve prostheses and who had relative contraindications to surgical valve replacement. The procedures were performed at Guy’s and St Thomas’ NHS Foundation Trust, London, United Kingdom. The primary diagnosis was variable with variable pulmonary artery morphology. The median age was 14 years (range 12-62 yrs). There was no mortality and no major morbidity, with satisfactory haemodynamic and angiographic outcomes in all patients. Post-procedural follow-up (median follow-up 8.5 months, range three to 15 months) with echocardiography and magnetic resonance imaging showed no restenosis or regurgitation with significant improvement in the right ventricular end-diastolic volumes.
Conclusions: Preliminary results show that the Venus P-valve can be safely and efficaciously used in dilated right ventricular outflow tracts of varying morphology. Rigorous trials will be required to evaluate this valve before more widespread use.
Abbreviations
CHD: congenital heart disease
CXR: chest X-ray
ECG: electrocardiogram
LPA: left pulmonary artery
MHRA: Medicines and Healthcare Products Regulatory Agency
MPA: main pulmonary arteries
MRI: magnetic resonance imaging
PA: pulmonary artery
PPVI: percutaneous pulmonary valve implantation
RPA: right pulmonary arteries
RVOT: right ventricular outflow tract
TTE: transthoracic echocardiogram
Introduction
Although surgical treatment of congenital heart disease (CHD) has evolved dramatically in recent decades, operations involving the right ventricular outflow tract (RVOT) can still result in chronic pulmonary regurgitation, necessitating surgical or percutaneous pulmonary valve implantation1.
Percutaneous pulmonary valve implantation (PPVI) has emerged as a successful alternative to surgical valve implantation in such patients2. However, the currently available balloon-expandable percutaneous valves are best suited for outflow tracts reconstructed with circumferential surgical tubes. Treatment of pulmonary regurgitation in the setting of previous RVOT patch augmentation has traditionally been limited to surgical replacement. This is mainly due to the limited potential size of balloon-expandable valves relative to often dilated outflow tracts. New techniques and technology are being developed to facilitate valve insertion in such cases. Outflow tract reducers and valves placed in a hybrid manner (with simultaneous plication of the outflow) are in current development3,4. Alongside this is the development of self-expanding percutaneous valves. The Venus P-valve® (Venus MedTech Inc., Hangzhou, China) is a new arrival, which consists of a nitinol stent and valve leaflets made from porcine pericardium. It may fulfil the needs of many patients and clinicians who are keen to avoid sternotomy and cardiopulmonary bypass for valve replacement.
Clinical experience with this new valve has previously been limited to a few cases performed in Asia5. We describe the first experience from Europe or North America implanting this new valve in five patients with different outflow tract pathologies. We also report the first use of a new modification of this valve, with a non-flared tubular design, in one of our patients.
Methods
Five patients underwent percutaneous Venus P-valve implantation between July 2014 and June 2015 in the congenital catheterisation laboratory at Evelina London Children’s Hospital, Guy’s and St Thomas’ NHS Foundation Trust, London, United Kingdom.
Patients were selected according to specific criteria for percutaneous pulmonary valve implantation in the context of this new valve technology (Table 1). These differ from the criteria for the Melody® (Medtronic, Minneapolis, MN, USA) and Edwards SAPIEN (Edwards Lifesciences Inc., Irvine, CA, USA) valves as the design of the Venus P-valve focuses on dilated outflow tracts, either native or with surgically constructed grafts.
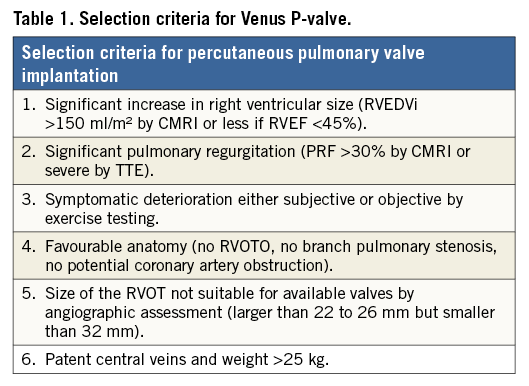
The indication for treatment may not have been pure regurgitation, rather a combination of regurgitation and stenosis, with suggestive symptomatology and serial exercise test results playing an important role in decision making.
The patients were counselled regarding the available treatment options including surgical pulmonary valve replacement as the standard of care in our institution. In these five cases, the surgical option was not preferred either due to the patient’s strong wishes or the fact that they had relatively increased risks with a surgical approach. Suitability for the Venus P-valve and valve sizing was based on a combination of non-invasive imaging (magnetic resonance imaging [MRI]) and diagnostic cardiac catheterisation.
The Venus P-valve does not yet have a CE mark. Therefore, exceptional approval authorisation (compassionate use) from the UK Medicines and Healthcare Products Regulatory Agency (MHRA) was obtained for each case.
PATIENTS
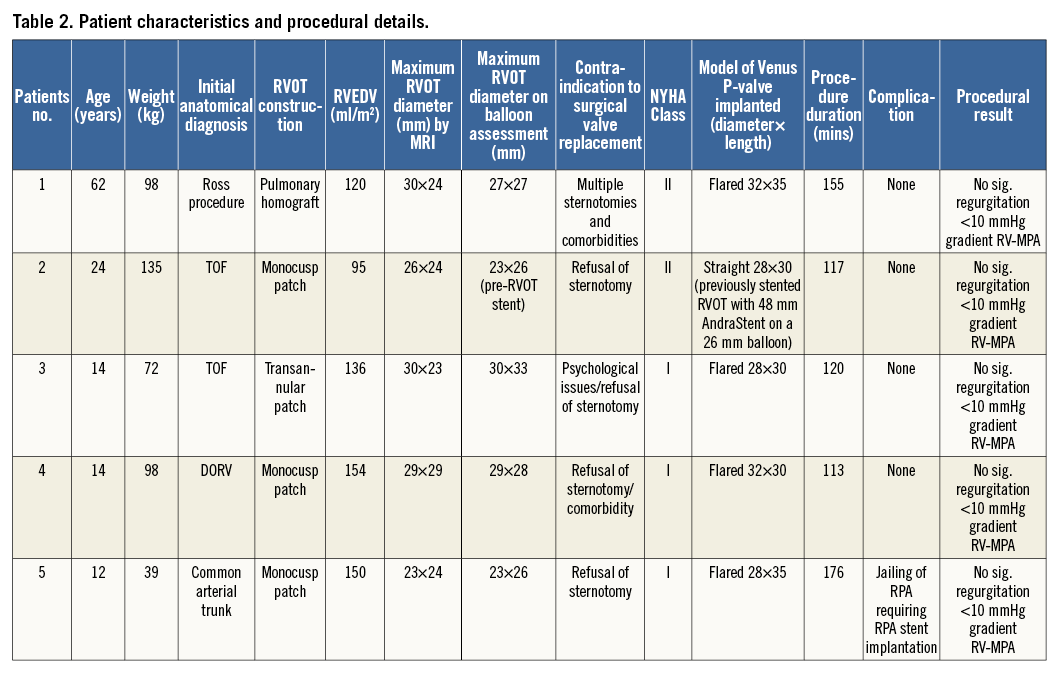
Patient characteristics and procedural outcomes are described in Table 2. The decision to consider the Venus P-valve was made in each case by taking into account a combination of surgical comorbidity risks, the wishes of the patients, and unsuitability for commercially available transcatheter valves. All the cases were discussed at a multidisciplinary surgical case conference before deciding on this treatment course.
INITIAL CARDIAC CATHETERISATION
Initial cardiac catheterisation was performed to determine suitability for Venus P-valve implantation by angiographic assessment according to a protocol derived from routine percutaneous valve work-up but adjusted to account for the differences attributable to the P-valve’s characteristics. A key part of this assessment was balloon occlusion angiography of the RVOT. After inflating a low-pressure 34 mm Amplatzer™ sizing balloon (St. Jude Medical, St. Paul, MN, USA) in the RVOT, an RV angiogram was performed simultaneously via a pigtail catheter introduced through a second venous sheath. This manoeuvre was aimed at fully delineating the muscular RVOT as well as the pre-existing native valve and main pulmonary arteries (MPA). Measurements of the sizing balloon were taken once we had ensured that the outflow tract was fully occluded with RV angiography. As per our usual practice, selective coronary angiography during inflation of a sizing balloon in the RVOT was also performed to allow risk assessment for coronary compression during valve implantation.
The length, diameter and design (flare or non-flared) of the individual valves were determined at this catheterisation procedure, allowing time to order a range of valve sizes for each case. We noted a significant difference between the MRI and sizing balloon diameter of the outflow tracts. This may relate to the expansile nature of the outflow tracts selected for the Venus P-valve cases and adds weight to the argument that careful balloon assessment of the outflow tract is an essential part of the overall process.
VALVE DESIGN (Figure 1)
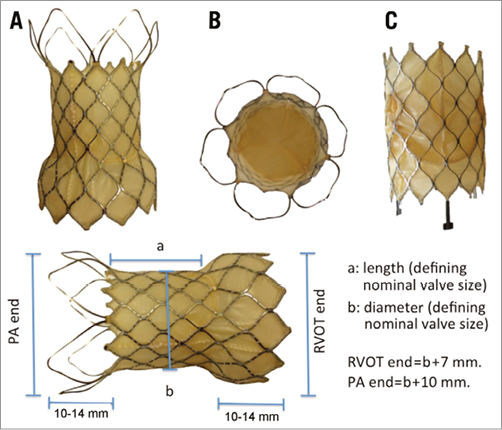
Figure 1. The Venus P-valve. A) The flared design of the P-valve with the flared covered RVOT end and uncovered flare at the pulmonary bifurcation. The stitching attaching the porcine pericardium to the nitinol frame can be seen. B) An en face view of the distal end of the flared prosthesis. C) The straight variation of the valve, designed specifically for use in patients who already have a stent in the RVOT. The measurements appended to the flared valve describe the relationship between the nominal measurements (length and width of the straight, covered, central region of the valve). The length of the flared segments depends on the width of the valve: 10 mm for the smaller valves and 14 mm for larger.
The skeleton of the Venus P-valve comprises a laser cut nitinol stent with leaflets made from porcine pericardium and hand-sewn to the self-expanding frame. The frame is anchored in the RVOT and distal MPA by flared proximal and distal sections. The proximal flare is completely covered by pericardial tissue, whilst the distal flare is an exposed, open-cell wire frame to prevent jailing of the pulmonary artery branches. The middle section contains the valve leaflets. The valve is also produced in a non-flared, straight design, which is potentially suitable for pre-stented outflow tracts.
The central (non-flared) portion of the valve is sized in 2 mm increment diameters from 22 to 36 mm, and in 25 mm, 30 mm and 35 mm lengths, accommodating a wide variety of size and morphology. The proximal (RVOT) flared segment diameter is 7 mm wider than the middle segment, and the distal flare is 10 mm wider. The length of the proximal and distal crowns depends on the diameter of the valves but is between 10 and 14 mm. The selected valve diameter is ideally 2-4 mm larger than the sizing balloon dimension during MPA balloon interrogation, whilst the selected length is matched, where possible, to the distance from the RVOT to PA bifurcation (Figure 2).
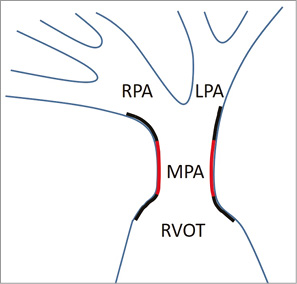
Figure 2. Schematic representing ideal positioning of a flared valve. This schematic shows the “ideal” position of the flared Venus P-valve after deployment. The black lines represent the flared portions and the red lines represent the central portion (with the valve positioned in the lower third of this). The flares positioned at the bifurcation point are uncovered, therefore providing free flow into both branch PAs, whereas the bottom flared area is covered, decreasing paravalvar leak (Figure 1). Both flares provide anchor points during deployment and afterwards.
Radiopaque platinum markers at the proximal and distal ends of the stent facilitate fluoroscopic location of the valve, particularly during deployment. There are two small hooks at the proximal end of the valve to attach it to the delivery system; however, these release as part of the deployment manoeuvre, without any specific manipulation. The delivery system comprises a 14 to 24 Fr valve housing, a 16 Fr 100 cm shaft, and a handle containing the deployment mechanism. The valve prosthesis is cooled in iced saline to stiffen the nitinol frame and allow crimping, using a combination of a crimping device and manual compression.
PROCEDURE DETAILS (Figure 3, Figure 4)
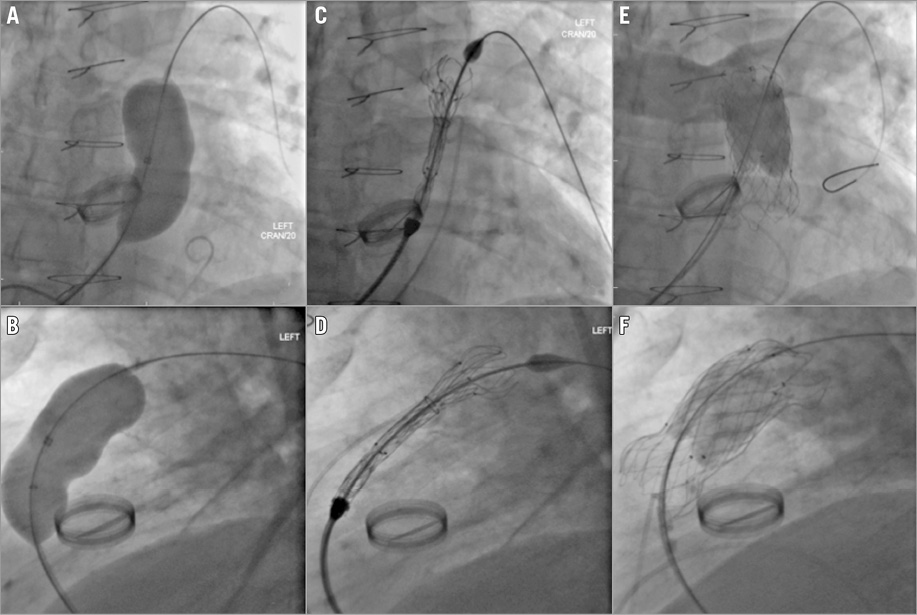
Figure 3. Implantation of the flared Venus P-valve in a 62-year-old patient. RVOT assessment and deployment of the flared Venus P-valve. A) & B) Balloon occlusion assessment of the RVOT. C) & D) Exposition of the distal end of the prosthesis. E) & F) The valve in final position with satisfactory check angiography.
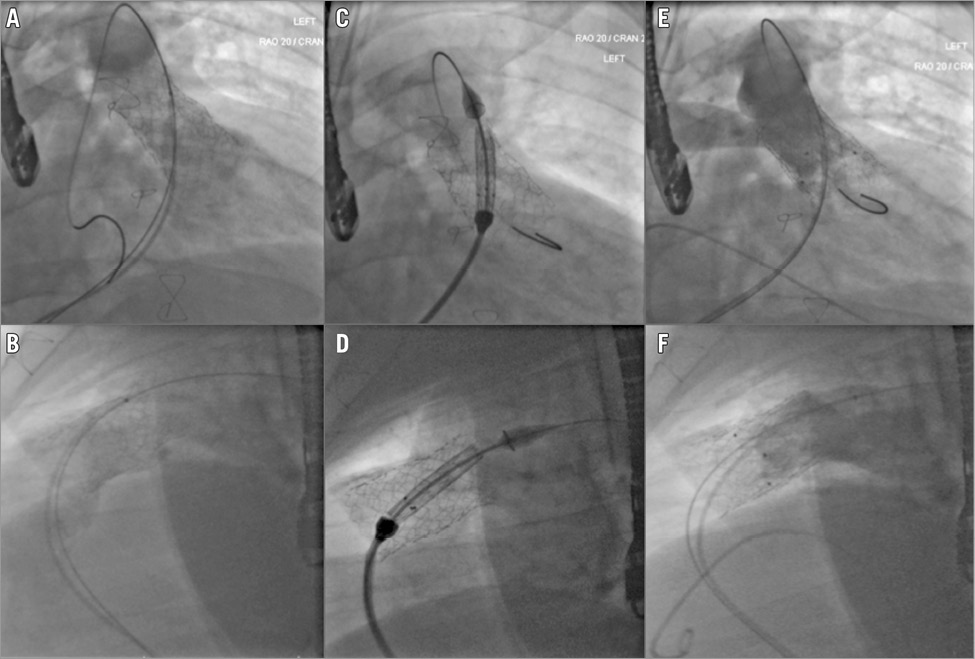
Figure 4. Implantation of the straight Venus P-valve in a 24-year-old patient with pre-stented RVOT. RVOT stent assessment and deployment of the straight version of the Venus P-valve. Deployment within a pre-stented RVOT is comparable to a balloon-expandable valve with no planned movement of the valve position during the deployment process. A) & B) Fluoroscopic assessment pre-stent. C) & D) Positioning of the valve prosthesis inside the stent, the black markers correlating to the lower extremity of the actual valve. E) & F) Angiograms confirming a satisfactory outcome.
The procedures were performed under general anaesthesia in the biplane cardiac catheterisation laboratory at the Evelina London Children’s Hospital at Guy’s and St Thomas’ NHS Foundation Trust. Access was gained under ultrasound guidance in both femoral veins, and arterial access provided continuous haemodynamic monitoring. The activated clotting time was maintained at greater than 220 seconds. Intravenous antibiotics were administered during the procedure.
Repeat diagnostic angiographic and haemodynamic measurements were performed at the start of each valve implantation procedure. A deep branch of the left pulmonary artery (LPA) was then accessed using a 6 Fr multipurpose catheter over a 0.035” hydrophilic Terumo guidewire (Terumo Corp., Tokyo, Japan), which was exchanged for a 260 cm long 0.035” Lunderquist® extra-stiff guidewire (Cook Medical, Bloomington, IN, USA), which was used for positioning and deploying the valve. The multipurpose catheter was passed across the tricuspid valve in a looped configuration to minimise the chance of entrapment of the valve apparatus.
The Venus P-valve was crimped onto the delivery system after rinsing for 10 to 15 minutes in a bath of freezing cold sterile saline. The delivery system was inserted through a 22-26 Fr GORE® DrySeal (W.L. Gore, Flagstaff, AZ, USA) delivery sheath and advanced over the stiff guidewire. We used the DrySeal system to minimise blood loss during equipment changes; we had a tendency to use a slightly larger than necessary DrySeal to allow for unforeseen problems, given the novel nature of the technology. Careful manipulation of the valve assembly was required to overcome any resistance faced around the intracardiac curves. The delivery system has a distal tapered “carrot” which aids progress into the proximal segment of the LPA where the distal flare is exposed. The valve is unsheathed slowly and carefully by clockwise rotation of a release wheel on the handle. After the distal flare has been exposed in the LPA, the system is slowly withdrawn to allow the flare to expand fully in the distal MPA. Periodic RV or MPA angiograms during valve deployment are required to ensure correct positioning. Haemodynamic compromise can be expected during the second half of the deployment procedure as the covered valve stent opens up in the MPA and RVOT causing temporary occlusion. Rapid exposure of the proximal portion of the valve assembly then completes the procedure.
Repeated pressure measurements along with RVOT and MPA angiograms were performed after successful valve deployment along with a transoesophageal echocardiogram to assess any residual stenosis, valve insufficiency or for any other possible complications.
Haemostasis of the large femoral venous access site was successfully achieved immediately in each case with a figure-of-eight suture6. Manual compression was used for the smaller venous access and the arterial access sites.
Intravenous antibiotics were given for the initial 24 hours post-procedure, followed by a five-day course of oral antibiotics as prophylaxis along with oral aspirin 5 mg/kg/day indefinitely.
Follow-up was organised after one and three months after discharge from the hospital. The follow-up included clinical evaluation, electrocardiogram (ECG), chest X-ray (CXR) and transthoracic echocardiogram (TTE). MRI, to check the integrity of the stent and the competence of the implanted valve, was arranged at two to three months following the procedure.
ADDITIONAL PROCEDURES AND COMPLICATIONS
Patient 2, who had previous MPA stenting performed with an AndraStent® (Andramed GmbH, Reutlingen, Germany) due to significant stenosis, had further stent dilation with a 28 mm Cristal® balloon (Balt, Montmorency, France) to optimise the outflow tract haemodynamics immediately before the valve insertion. Although this patient would potentially have been suitable for a 29 mm SAPIEN valve, we felt that redilation of the stent at the time of the percutaneous valve implantation may have resulted in an unsuitable landing zone. Therefore, we opted to plan the procedure for Venus P-valve and not risk a failed implantation with the SAPIEN.
In patient 5, the pulmonary artery anatomy was complex because of the previous repair of the common arterial trunk. There was significant asymmetry between the right pulmonary artery (RPA) and LPA and an unusual take-off angle of the RPA origin (Figure 5). The P-valve was deployed; however, on check angiography, it was apparent that the RPA origin was occluded by the distal part of the Venus P-valve. The valve position was successfully adjusted by applying traction on the lower edge of the flare with biopsy forceps. After this adjustment, there was good flow into the RPA, but there was origin stenosis of the RPA with a gradient of 30 mmHg associated with the position of the distal flare. A 26 mm long LD Max™ stent (Covidien/Medtronic) crimped onto a 15 mm balloon was implanted in the proximal RPA, through the uncovered strut of the valve frame, and a satisfactory haemodynamic (0 mmHg gradient) and angiographic result was obtained. There was no haemodynamic compromise. The patient recovered without consequence and was discharged as planned.
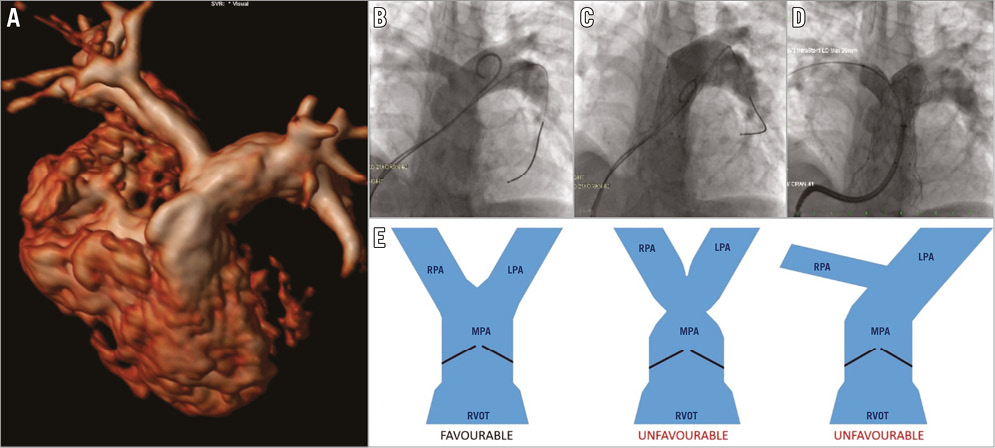
Figure 5. Implantation of the flared Venus P-valve in a 12-year-old patient with asymmetric branch pulmonary arteries after repair of truncus arteriosus. Pulmonary artery morphology. Suitability for Venus P-valve implantation may partly rely on the morphology of the pulmonary bifurcation. Angiograms from case 5 in our series demonstrate occlusion of the RPA (C) which required stent implantation to secure patency (D). This arose due to the asymmetric morphology and angulation of the bifurcation (A & B). The schematic (E) demonstrates the ideal pulmonary bifurcation morphology contrasted with two situations which make delivery of the distal flare of the Venus prosthesis difficult. The asymmetry shown in the right-hand image may equally be a mirror image with a small and angulated LPA origin.
Results
There was no mortality and no major morbidity associated with the Venus P-valve implantation. Post-procedural angiography revealed trivial or absent pulmonary regurgitation. There were no haemodynamic concerns during direct measurement after implantation or by echocardiographic assessment. The mean pullback gradient across the implanted valve was 6 mmHg. TTE performed 24 hrs after the procedure showed no evidence of valve regurgitation. There was no gradient across the valve either by colour Doppler or continuous wave Doppler interrogation. Anteroposterior and lateral chest X-rays showed stable valve positions with no obvious fractures or distortion.
All the patients were extubated in the catheter laboratory and remained stable throughout the hospital stay with no evidence of arrhythmias. The first patient stayed in the hospital for three days to receive unfractionated heparin until the international normalised ratio (INR) was >2 with warfarin, because of a mechanical aortic valve. He received three days of intravenous antibiotics, due to a history of previous infective endocarditis. The second patient had a past history of pulmonary embolism and was kept in the medical ward for four days to re-establish warfarin therapy. The other patients were loaded with aspirin followed by a maintenance dose of 5 mg/kg/day. They were all kept as inpatients for three days after the procedure due to the novel nature of the intervention. Oral antibiotics were prescribed in each case to complete a total of five days treatment.
Follow-up MRI results
Figure 6 outlines the CMRI results preoperatively and two to three months after Venus P-valve implantation. Three of the five patients have so far had follow-up MRI scans. The figure shows significant improvements in the indices after treatment.
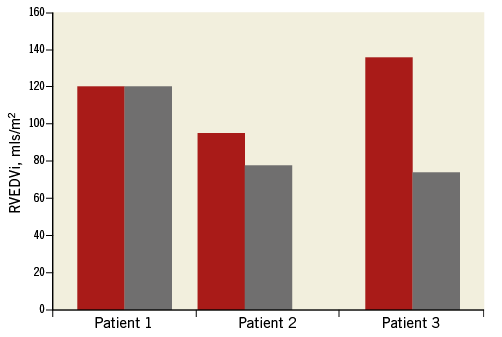
Figure 6. Improvement in MRI-derived RVEDVi following Venus P-valve implantation. MRI-derived RVEDVi pre and post Venus P-valve implantation. The follow-up MRI scans were performed within six months of valve implantation. Patient 2 initially had significant valvar stenosis before RVOT stenting, accounting for the relatively low pre-intervention RV volume.
Discussion
PPVI is an exciting and rapidly expanding area in the field of interventional cardiology. Although particularly prevalent in patients following tetralogy of Fallot repair, the pathophysiology and techniques are applicable in many patients with native or iatrogenic RVOT pathology6,7.
The optimal timing of pulmonary valve replacement is still a topic of debate, but it has become clear that restoration of pulmonary valve competence improves the right ventricular (RV) function and the clinical symptoms, if performed before irreversible ventricular damage occurs8,9. The advent of non-surgical approaches may have reduced the threshold for intervention due to the decreased morbidity of percutaneous techniques. A major limitation of PPVI has been its applicability in native RVOTs7,10,11. These difficulties ultimately exclude approximately 85% of patients requiring reintervention for pulmonary regurgitation and a dysfunctional RVOT12.
The two currently approved percutaneous valves are the Melody and the Edwards SAPIEN transcatheter heart valves. Despite reports of success in the use of these balloon-expandable valves in native RVOTs, the limiting factor is their maximum potential size in relation to severely dilated native outflows, and therefore they are mainly implanted into surgically constructed outflow tracts13,14. The maximum diameter of a stable landing zone for the Melody and SAPIEN valves is 22 mm and 25 mm, respectively, although the recently released larger 29 mm SAPIEN valve has also been used in the pulmonary position and may allow a stable valve implant in lumen of up to 27 mm diameter8.
The novel transcatheter self-expanding Venus P-valve has been developed specifically for percutaneous use in the native RVOTs to overcome the challenge of the larger outflow tracts.
The flared ends of the Venus P-valve are designed specifically as anchoring points to provide stability in large pulsatile outflows. Hence, it can be utilised in outflow tracts up to a diameter of around 32 mm, significantly expanding the applicability of PPVI in these patients15-17. In addition, it reduces the need for pre-stenting, which may reduce the risks, costs, procedure time, and radiation exposure.
The straight design of the P-valve shares more characteristics with pre-existing balloon-expandable percutaneous valves and benefits from a pre-stented landing zone.
The nitinol framework of the Venus P-valve has low radial force which may reduce the risk of coronary artery compression, a major concern with currently available valves14,18. Nitinol should allow good conformability of the bioprosthesis in the RVOT, minimising tension on the framework and therefore reducing the incidence of or even preventing stent fractures18. The flexible nitinol frame also gives more confidence for valve positioning in outflows which have significant pulsatility, a feature which is a relative contraindication for implantation of a balloon-expandable valve.
The Venus P-valve is constructed with porcine pericardium as opposed to bovine internal jugular vein. Porcine pericardium is known to provoke a significant immune response in human subjects, which may play a part in the development of early valve dysfunction in other valve technologies19-21. We will need to be vigilant for signs of early leaflet dysfunction with the Venus P-valve.
The published experience with the Venus P-valve is minimal. In a clinical series reported by Cao et al, five patients with severe PR and native RVOTs of diameter 31.8±5.1 mm were selected. Follow-up three months after valve implantation demonstrated a significant improvement in pulmonary regurgitation, right ventricular volumes, and NYHA class5. There were no reports of stent fracture or valve dysfunction at early follow-up.
The result from our small series, with successful implantation of the Venus P-valve in all five patients with different pathologies, is promising. There were no severe adverse events. Short-term follow-up showed minimal or zero pulmonary regurgitation, a marked reduction in RVEDV and no stent fractures.
The experience with patient 5 represents a now recognised morphological concern, which will need to be considered in patient selection. Significant LPA/RPA asymmetry or an asymmetric PA bifurcation may cause this valve to deploy in a less than ideal configuration. In patient 5, both of these factors resulted in a deployment which was too distal. The covered part of the valve stent therefore occluded the RPA. The valve had to be repositioned, which required a significant amount of traction force. Even after repositioning the valve, the covered stent still partially occluded the RPA origin and so a stent had to be deployed in the proximal RPA. This is an important part of our learning curve. In retrospect, and also for the future, a shorter length of the valve could have been used or perhaps this patient could have been excluded because of the morphology. The applicability of TOE is uncertain, but we elected to place a TOE probe which was quite useful in assessing the valve function immediately post implant. Its main use, however, has been to provide prophylactic imaging capability in the event of an unexpected complication, given the novel nature of the technology.
Limitations
Despite the overall success, our data are limited by small patient numbers and the short duration of follow-up. Three of our five patients have so far completed one year of follow-up, with MRI and echocardiographic results indicating success. Further studies and long-term follow-up data are required in order to evaluate the function and durability of the Venus P-valve. It is difficult to demonstrate a significant change in the symptoms or NYHA class during this short follow-up duration. In order to demonstrate symptom change objectively, performing serial exercise testing may be beneficial. None of the patients reported any palpitations or demonstrated rhythm disturbance on ECG immediately post procedure or during the follow-up.
Conclusions
The new self-expanding Venus P-valve exemplifies the marked progress made over the last decade in the field of PPVI. It is promising, but challenges still exist including reducing the delivery system size further to aid implantation in smaller children. We are also beginning to recognise morphologies in which Venus P-valve implantation may be challenging or contraindicated and are beginning some computerised modelling to assess this. Ultimately, the success of PPVI hinges on the ability to extend the application of this technology to fulfil broader clinical needs. The Venus P-valve is another step along the road.
| Impact on daily practice The Venus P-valve gives the potential for percutaneous pulmonary valve implantation in a much wider group of patients than is currently feasible. Initial results suggest that it is a safe and efficacious technology, but rigorous trials are necessary before more widespread use. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

