Abstract
Aims: To present our experience of ‘first-in-man’ implantation of a new percutaneous pulmonary valve into a dilated pulmonary trunk, using patient specific data to influence the design of the device and ensure patient safety.
Methods and results: A 42-year-old with severe pulmonary insufficiency underwent computed tomography assessment of his pulmonary trunk. This information was utilised to create computer and rapid prototyping models that were used to customise and test the device, which was subsequently implanted into the patient. Following the procedure, the clinical, haemodynamic and morphological success of this approach was determined.
The new device was safely and successfully implanted as predicted by the pre-procedural modelling. There was excellent device stability, no stent fractures, no pulmonary incompetence and only trivial para-device leak at six months follow-up. The patient described marked symptomatic improvement.
Conclusions: Safe, effective percutaneous pulmonary valve implantation is possible in a patient with a dilated, native pulmonary trunk. Our methodologies, which have evolved as a direct result of recent advances in four-dimensional imaging techniques, challenge the conventional stepwise pathway of bench and animal testing prior to human application, and may be safer and more relevant, potentially reducing the number of animal experiments necessary for testing new medical devices.
Introduction
In 2000, we reported the ‘first-in-man’ implantation of a percutaneous heart valve – placing a pulmonary valve into a dysfunctional right ventricle to pulmonary artery prosthetic conduit1. Over the following decade, this technology has been accepted into clinical practice, with over 1,000 devices now implanted throughout the world, with CE marking in Europe and Canada, and the Food and Drug Administration (FDA) approval in the USA – the Melody™ (Medtronic Inc., Minneapolis, MN, USA).
Using this percutaneous device, patients have been treated safely to relieve both pulmonary stenosis and regurgitation2-6. However, because of the wide variation in patient morphology, size, and dynamics of the right ventricular outflow tract (RVOT)/pulmonary trunk, only 15% of patients with a haemodynamic and clinical indication for pulmonary valve replacement can be treated with the current device7,8. Thus, 85% of patients with pulmonary dysfunction still require open-heart surgery for treatment.
The majority of these patients are those with dilated, dynamic RVOT/ pulmonary trunk anatomy (patients with Tetralogy of Fallot and previous RVOT patches) in whom the current percutaneous device is too small. Importantly, for this morphological problem, animal testing is of very limited use, as there are no models that encompass the wide variations of anatomy seen in these patients with congenital heart disease8. Hence development of a new device to deal with the clinical problem and its translation into man is difficult using the conventional pathway of bench followed by animal testing and ultimately ‘first-in-man’ application.
In this paper, we describe a ‘first-in-man’ procedure of a new percutaneous pulmonary heart valve device. We have used advanced cardiovascular imaging, in combination with patient-specific computer modelling to address the challenges identified above to safely introduce the device into early clinical practice.
Methods
Over the last three years, we have been developing a new percutaneous pulmonary valve device for implantation into the dilated RVOT/pulmonary trunk in collaboration with Medtronic, Inc. The device consists of an hourglass-shaped, nitinol covered stent, with a porcine pericardial valve sewn into the central portion (Figure 1).
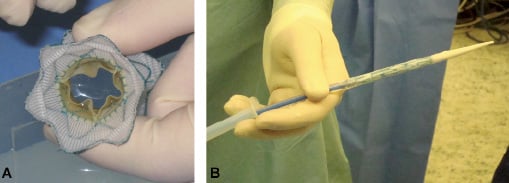
Figure 1. New device. (A) An end-on-view of the new device showing the Nitinol stent with graft covering and the open pericardial valve leaflets. (B) The delivery system with the device crimped and loaded onto the distal end (right side of picture).
Conventional animal testing and bench testing had been undertaken for this device and demonstrated a good performance in the animal model9. During the final stages of this preclinical testing, a patient was presented to our institution requiring RVOT dysfunction treatment.
The patient (a 42-year-old man with congenital heart disease) had previously undergone four open-heart operations and two additional thoracic procedures. Most recently, he had undergone mitral valve replacement but without the planned revision of his regurgitant pulmonary valve, due to technical difficulties related to mediastinal adhesions and an already prolonged bypass time. Following this operation, the patient remained very symptomatic with worsening right heart failure, secondary to severe pulmonary insufficiency. Exercise capacity was limited to walking less than 20 yards and he had gross peripheral oedema with ascites.
Cardiothoracic surgery was considered too high risk. Furthermore, the RVOT/pulmonary trunk was too dilated for the available percutaneous pulmonary valve device – Melody™ (suitable for conduits 14-22 mm in diameter). Therefore, the patient was considered for implantation of the new device.
Imaging and modelling assessment
Four-dimensional (4D) contrast enhanced, ECG-gated cardiovascular computed tomography (CT) was performed to acquire three-dimensional (3D) images over 10 frames of the cardiac cycle (Definition, Siemens, Erlangen, Germany). The CT data was used to reconstruct 3D volumes of the implantation site and measure its deformation throughout the cardiac cycle. The 3D volumes were divided into sections (Figure 2A) and diameter changes over the cardiac cycle were measured at each section (Figure 2B). Based on these reconstructions, finite element models and 3D rapid prototype polymer models of the RVOT, pulmonary trunk and proximal branch pulmonary arteries were created, as described by us previously7. Multiple device shapes and sizes with varying wire stiffness and configurations were tested in the rapid prototyping models and using finite element analysis to optimise the anatomical results in the specific patient.
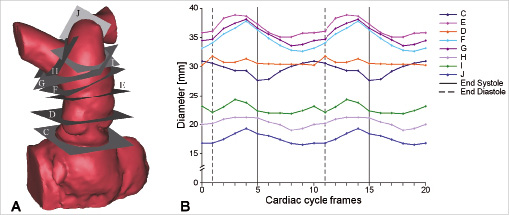
Figure 2. RVOT dynamics. (A) 3D volume reconstruction of the RVOT, pulmonary trunk and pulmonary bifurcation from 4D CT. Dynamic changes were measured in the eight planes shown (C-J). (B) Diameter changes over the cardiac cycle measured in the planes shown in (A). The diameter was calculated from the perimeter changes of each section, to take into account the eccentric shape of some regions.
The final device (40 mm diameter in the distal and proximal ring, 22 mm diameter in the central portion holding the valve) underwent acute and chronic animal tests and bench testing, and these results were submitted to the Medicines and Healthcare products Regulatory Agency (MHRA) for risk assessment of this individual case. Despite lack of full supportive pre-clinical data and because there was no alternative for this patient, MHRA concluded that there was sufficient evidence to support the use of the new device on humanitarian grounds, (the benefits outweighed the risks), and the device was offered as a treatment. The patient gave informed consent for the procedure and for the anonymous use of his data and images for publication. Ethical and industrial approval to implant a novel custom-made device was obtained.
Device implantation
Under general anaesthesia, catheterisation was performed through the right femoral vein. The patient was heparinised. Angiographic studies (Video 1), haemodynamic assessment and balloon sizing (Video 2) of the pulmonary trunk were performed to identify the optimal site for device implantation. After positioning a stiff guide wire in the left pulmonary artery, the self-expanding valved stent-graft was front-loaded onto the custom made delivery system (Medtronic Inc., 24 Fr external diameter at the tip, Figure 1). The system was then advanced along the guide wire (ultrastiff Back-up Meier wire, Boston Scientific/Medi-tech, Watertown, MA, USA) to the dilated, native pulmonary trunk where the stent-graft was uncovered and released. Following device implantation, angiographic studies (Video 3) and haemodynamic assessment were performed.
Follow-up
The patient was followed up clinically, with echocardiography (performed immediately after, and then at three days, one month, three and six months following the procedure), with 4D contrast enhanced, ECG-gated CT (three days and three months post-procedure), and with non-contrasted biplane fluoroscopy (anteroposterior and lateral projections, one month, three and six months after implantation). Three-dimensional analysis of the reconstructed CT data was used to assess the deformations of the device and implantation site over the cardiac cycle and to visualise for stent fractures.
Results
Pre-procedural assessment
The 4D CT dataset facilitated quantitative assessment of the dynamic variation in pulmonary trunk morphology throughout the cardiac cycle (Figure 2 & 3A). It was confirmed that the pulmonary trunk (minimum dimension=28 mm) was too large for implantation of a Melody™ device, but compatible with the new device technology.
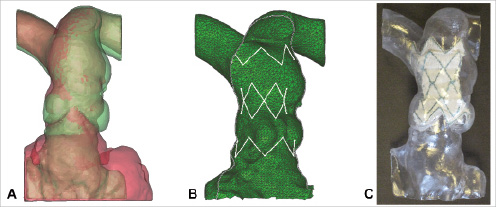
Figure 3. Modelling. (A) Superimposed diastolic (red) and systolic (green) 3D volume reconstructions of the patient anatomy from 4D CT. Note how the pulmonary trunk has its maximal dimensions in systole, whilst the underlying RVOT is at its smallest. (B) Finite element model of the contact between the stent-graft (white) and the patient anatomy in systole (green). (C) Plastic rapid prototype model of the patient anatomy with the final device design inserted.
The patient’s measurements in combination with the results of the deployment tests in the 3D rapid prototyping models were used to customise the size of the available device. The finite element analysis subsequently identified definitive areas of contact between the computer simulated stent-graft struts of the customised device and implantation site, predicting likely stability and safe anchoring in vivo (Figure 3B). Trial implantation of the final device into the rapid prototype, polymer models confirmed that the chosen design should be optimal for the patient (Figure 3C).
The rapid prototyping models enabled simulation of the clinical implantation procedure and helped the implanters (PB, MC) decide the approach for optimal device delivery (guide wire in the left pulmonary artery, device deployment with the distal portion just within the left pulmonary artery, pull-back of the device via delivery system retrieval for the correct positioning of the distal part into the pulmonary trunk).
Device implantation
At catheterisation, balloon sizing of the pulmonary trunk confirmed that the CT and model dimensions were a true representation of the patient’s anatomy (mid-pulmonary trunk – balloon diameter= 35 mm, CT/model dimensions=33 mm [diastole] – 37 mm [systole]). This gave the implanters the confidence to proceed. The device was uncovered at the origin of the left pulmonary artery and pulled back slightly until the morphological appearance was as expected from the pre-procedural trial implantation. The position of the device was confirmed by fluoroscopy (Figure 4A), and the competency of the pulmonary valve was demonstrated with trans-oesophageal echo. Haemodynamically, systolic right ventricle (RV) pressure was unchanged (35 vs. 34 mmHg), demonstrating that the device did not cause pulmonary trunk or branch pulmonary artery obstruction. The diastolic pulmonary pressure increased (5 vs. 12 mmHg), consistent with restoration of pulmonary valvular competency. Ascending aortic pressures improved (89/47 vs. 103/53 mmHg) possibly as a result of improved left ventricle (LV) filling and ejection, secondary to increased pulmonary blood flow. The patient recovered quickly following termination of general anaesthesia.
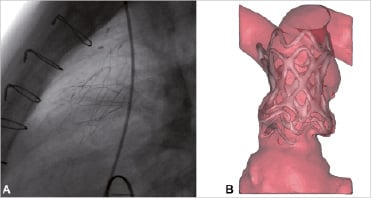
Figure 4. Post-implantation. (A) Lateral x-ray fluoroscopy view of the device after final positioning. (B) Frontal view of 3D volume-rendered CT reconstruction two days after stent-graft implantation.
Post-procedural follow-up
The patient was symptomatically improved following the procedure, with a subjective improvement in exercise capacity (now able to walk two miles) and less discomfort from hepatic distension. Echocardiography on day three showed no valvular regurgitation and mild para-device leak, reduced to trivial at the six month review. The position, shape and safe anchoring of the device were confirmed on CT both at three days and three months after the procedure (Figure 4B, Video 4 & 5). CT at three months showed reduction in the amount of pulmonary incompetence (RV stroke volume = 148 vs. 90 mL/beat) and RV end-diastolic volume (308 vs. 237 mL). The LV stroke volume was increased from 67 mL/beat to 82 mL/beat. No fractures of the stent struts (examined at fluoroscopy) have been detected to date. The patient remains well 10 months following the procedure.
Discussion
Over the last three years we have been developing new percutaneous valve technology to treat the dilated, native pulmonary trunk – a development that would potentially extend trans-catheter therapy to all patients requiring pulmonary valve replacement. In this study, we report the first clinical application and our technical strategy for the introduction of this new device in a patient for whom there was no available alternative. Successful, safe implantation was achieved by utilising an integrated, as opposed to stepwise approach to pre-clinical and clinical testing, using patient imaging data, computer modelling and biomedical engineering to influence the final device design for implantation.
For our specific patient, dynamic measurements from 4D CT data and computer simulation enabled determination of the correct device size and confirmed that the device would be anchored adequately in the pulmonary trunk throughout the cardiac cycle. 3D rapid prototype plastic models allowed the interventional cardiologists to handle the 3D anatomy of the implantation site, informing the decision to place the guide wire in the left pulmonary artery and to practise the implantation. Visualising the optimal result in the model cued the slight repositioning of the device when the procedure was performed in vivo. Employing this methodology ensured procedural success.
Whilst such a labour intensive method, which resulted in modifications in the device design prior to this first human case, was essential to enable us to safely transfer this new technology into early clinical practice, this approach would not be necessary, or sustainable, for each patient in routine clinical practice. Ultimately, the aim for this new percutaneous pulmonary valve technology would be to have a small number of ‘off-the-shelf’ devices with varying sizes and shapes, which would be suitable for the vast majority of patients. The implantation of this new device represents the first step towards the percutaneous treatment of the dilated pulmonary trunk. Conservative estimates suggest that over 10,000 such devices would be implanted each year throughout the world once the technology is routinely available.
The incorporation of clinical data into pre-clinical testing may be useful for ‘first-in-man’ applications of other valve technologies, and, more broadly, for developing other cardiac and non-cardiac devices. For example, in our own initial experience with the Melody™ device, bench testing predicted that the valve would become regurgitant over time, and that the stent, in which the valve is fixed, would last for millions of cycles with no clinical concerns. However, our clinical experience was in fact the opposite, with good valvular function5, but a 20% incidence of stent fractures10, clearly showing that bench and indeed animal testing cannot always foresee the potential problems that may arise in the clinical setting. Similarly, though thousands of successful percutaneous aortic valve replacements have been performed worldwide following the first implantation by Cribier in 200211, the early clinical experience was accompanied by morbidity and mortality. The lack of relevant animal models, that would have made pre-clinical investigation more robust, potentially contributed to these early problems and limited the clinical indication of this transcatheter procedure. Such issues may have been overcome by the modelling pathways outlined in this study.
Although implantation of the new device was successful using the methodology that we have outlined and the accuracy of pre-procedural modelling and measurements was confirmed by balloon-sizing at the time of the procedure, the use of in vitro analysis to mimic the in vivo situation should be interpreted with caution until larger studies are performed to confirm our findings.
With this case, we have carried out the ‘first-in-man’ implantation of a new percutaneous valved device into the dilated pulmonary trunk and presented an innovative strategy that may enhance the safe introduction of new medical devices into clinical practice. Ultimately, this device may provide a percutaneous strategy for the majority of patients who require pulmonary valve replacement, thus reducing the number of surgical interventions required by patients over their lifetime. Furthermore, advances in imaging and engineering could shorten the time for device development, minimise animal experimentation, and ensure greater patient safety during the delicate phase of testing clinical feasibility of novel device technologies. Importantly, these methodologies do not replace the need for bench and animal testing, but should enable disease-specific data from patients to be considered when planning these phases of development, and may be applicable to a wide range of devices in medicine.
Acknowledgments
This work was supported by the Royal Academy of Engineering/EPSRC, the British Heart Foundation, the European Union – Health-e-Child initiative and the UK National Institute of Health Research. The authors would like to thank Medtronic Inc. for their support during this case.
Online data supplement
Video 1. Angiogram pre-implantation – frontal view.
Video 2. Balloon sizing of the patient’s pulmonary trunk – lateral view.
Video 3. Angiogram post-implantation – frontal view.
Video 4. 4D volume-rendered CT reconstruction two days after stent-graft implantation – frontal view.
Video 5. 4D volume-rendered CT reconstruction two days after stent-graft implantation – lateral view.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1.
Moving image 2.
Moving image 3.
Moving image 4.
Moving image 5.

