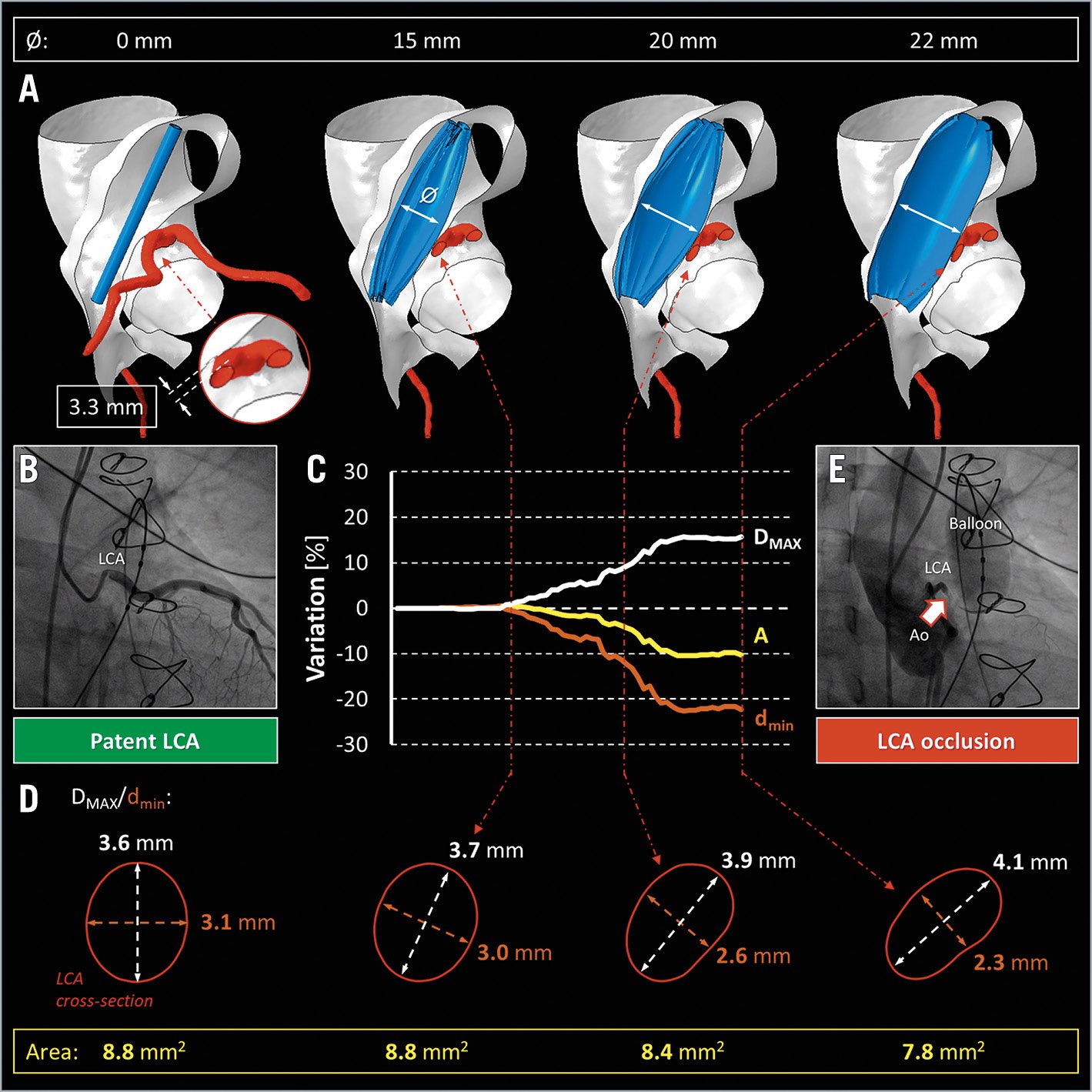
Figure 1. Numerical prediction of coronary artery occlusion. A) Still frames of the balloon angioplasty virtual reproduction up to 22 mm of balloon diameter (Moving image 2). B) Angiographic inspection of LCA patency (Moving image 1). C) Time course of the percentage variation in LCA dimensions, during virtual balloon inflation. D) Geometrical rearrangement of the LCA configuration on a single cross-section. E) Balloon angioplasty, during diagnostic cardiac catheterisation, confirming LCA compression (Moving image 3). Ø indicates the diameter of the inflated balloon. A: aortic cross-sectional area; Ao: aorta; DMAX: maximum LCA diameter; dmin: minimum LCA diameter; LCA: left coronary artery
A 40-year-old man, with a previous Ross procedure, presented with severe stenosis of a pulmonary homograft and was considered for transcatheter pulmonary valve implantation (TPVI). Three-dimensional (3D) anatomical reconstruction from preoperative computer tomography (CT) revealed a left coronary artery (LCA) in close proximity (3.3 mm) to the oval-shaped homograft (Figure 1A), while angiographic inspection confirmed coronary patency (Figure 1B, Moving image 1). To investigate the risk of LCA compression, a life-threatening TPVI complication, we exploited a CT-based patient-tailored finite element (FE) analysis1 (Figure 1A). This approach can reproduce balloon angioplasty virtually (Moving image 2), with a 22 mm diameter balloon in the right ventricle outflow tract (RVOT). When the balloon was inflated to 15 mm in diameter (Figure 1C), there was no impact on maximum (DMAX) and minimum (dmin) diameters (Figure 1D) of the LCA cross-section. Conversely, further balloon inflation, up to 22 mm in diameter, resulted in direct interaction of the homograft with the LCA, creating an elliptical cross-section due to a 16% increase in DMAX and a concomitant 22% reduction of dmin (Figure 1D); a 10% reduction of LCA lumen cross-sectional area (A) was noted. The numerical FE prediction proved to be consistent with intraprocedural fluoroscopy (Figure 1E): homograft angioplasty and simultaneous LCA angiography showed that coronary occlusion arose when the balloon diameter increased above 17-18 mm (Moving image 3). Hence, FE analysis effectively predicted the potential risk of coronary occlusion revealed by cardiac diagnostic catheterisation. However, given the unpredictable heterogeneity of the tissue and its degree of degeneration, the FE approach can only approximate the actual mechanical response of the homograft to balloon dilation. Despite this limitation, FE analysis provides a complementary strategy to in vivo measurements to assess TPVI feasibility.
Acknowledgements
This work was supported by the IRCCS Policlinico San Donato, a Clinical Research Hospital partially funded by the Italian Ministry of Health.
Conflict of interest statement
F. Pluchinotta has recently been employed by AstraZeneca, outside the submitted work. This work was conceived and created while she was working as a research physician in IRCCS Policlinico San Donato from 2010 to March 2020. F. Pluchinotta has no financial arrangement with products figuring in the submitted manuscript or with a company making a competing product. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Angiography of the left coronary artery showing vessel patency.
Moving image 2. Finite element reproduction of the homograft balloon angioplasty and prediction of the direct interaction of the homograft with the left coronary artery during balloon inflation.
Moving image 3. Homograft balloon angioplasty test during cardiac catheterisation revealing left coronary artery occlusion at the fully expanded, i.e., 22 mm diameter, balloon configuration.

