The patient is a 39-year-old female. She was initially diagnosed with a pulmonary valvular stenosis at the age of eight. At the age of 15, the systolic pulmonary gradient was measured at 70 mmHg by echocardiography and, in addition, there was an atrial septal defect (ASD) of the secundum type. She underwent cardiac surgery, during which the ASD was closed by direct suture technique, the pulmonary leaflets resected and the right ventricular outflow tract was enlarged by a transannular pericardial patch. In 2002 she was re-operated due to recurrence of symptoms with a 23 mm pulmonary homograft. Over time the homograft developed significant stenosis and regurgitation with clinical symptoms of heart failure and in 2011 she was in NYHA Class III-IV.
Following a multidisciplinary conference she was recommended transcatheter treatment with a 26 mm SAPIEN XT valve (Edwards Lifesciences, Irvine, CA, USA) using the 18 Fr NovaFlex delivery catheter (Edwards Lifesciences) and the split sheath introducer in a first-in-man treatment of a pulmonary conduit with severe regurgitation. The patient consented to two doctors on two occasions who described the procedure as off-label, first-in-man and potentially harmful.
In November 2011 the procedure was uneventfully performed, with an initial sizing balloon followed by pre-stenting with a 36 mm long Max™ LD stent (ev3 Endovascular Inc., Plymouth, MN, USA) and the valve implantation (Moving image 1-Moving image 6). The final minimal diameter of the skirt of the valve was 21 mm (Online Figure 1).
Follow-up ultrasound demonstrated a severe pulmonary regurgitation and a mild stenosis with a systolic pressure gradient of 29-42 mmHg. Visualising the actual cause of malfunction was not possible with echo, MRI or multislice CT (Online Figure 2). Redo surgery took place in November 2012 with excision of the old conduit and the SAPIEN XT valve. A biological stentless RVOT elan™ 23 mm (Vascutek, Inchinnan, Renfrewshire, UK) was used. The explanted Edwards valve had two of the three leaflets fully open and stuck/pinched between the Max™ LD stent and the Edwards XT valve´s scaffold (Figure 1). The Edwards SAPIEN XT demonstrated early valve failure which raises serious concerns about sizing, pre-stenting and type of pre-stent.
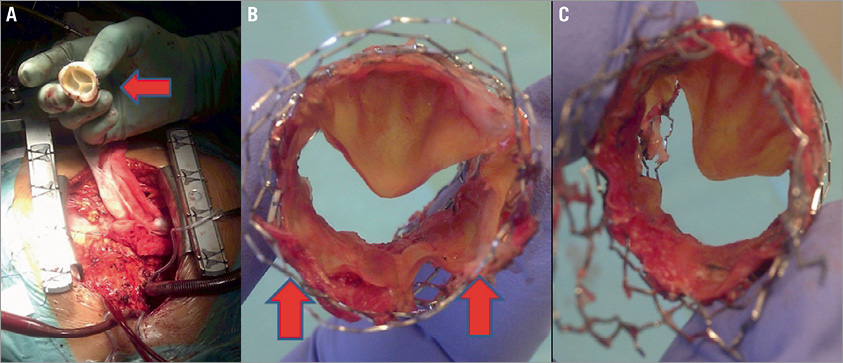
Figure 1. A) Photograph during cardiac surgery demonstrating the new elan™ stentless conduit. B) The explanted SAPIEN XT valve seen from above demonstrating the two stuck leaflets (red arrows) between the Max™ LD stent and the SAPIEN XT scaffold. C) The valve seen from below.
Conflict of interest statement
G. Olivecrona has been a proctor for Edwards Lifesciences in the USA, 2012. The other authors have no conflicts of interest to declare.
Author affiliations
1. Department of Coronary Heart Disease, Skane University Hospital, Institution of Clinical Sciences, Lund University, Lund, Sweden; 2. Center for Medical Imaging and Physiology, Skane University Hospital, Department of Diagnostic Radiology, Institution of Clinical Sciences, Lund University, Lund, Sweden; 3. Department of Heart Failure and Valve Disease (GUCH section), Skane University Hospital, Institution of Clinical Sciences, Lund University, Lund, Sweden; 4. Department of Thoracic Surgery, Anaesthesia and Intensive Care, Skane University Hospital, Institution of Clinical Sciences, Lund University, Lund, Sweden
Moving images
Moving image 1. Initial pulmonary angiogram using a 7 Fr MULTI-TRACK™ catheter (pfm medical ag, Cologne, Germany).
Moving image 2. Balloon dilation with a 24×50 mm NuMED balloon (NuMED, Denton, TX, USA) and simultaneous coronary angiogram.
Moving image 3. Pre-stenting with a 36 mm long Max™ LD stent crimped on a 24 mm BIB balloon.
Moving image 4. A 26 mm SAPIEN XT slowly implanted.
Moving image 5. Post-dilation of the valve using the valve balloon.
Moving image 6. Final pulmonary angiogram demonstrating a grade two central leakage.
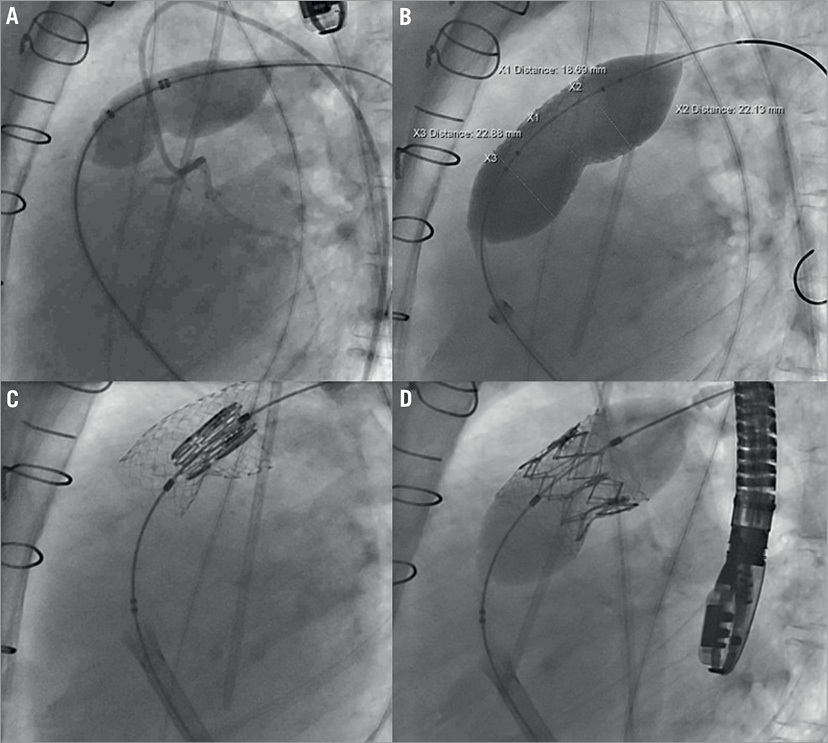
Online Figure 1. Angiographic images. A) The stenosis in the homograft was predilated several times with a 24×50 mm balloon. During inflation simultaneous coronary angiography was performed and revealed no signs of coronary compression. B) The landing area for the SAPIEN XT valve stented with a 36 mm long Max™ LD stent mounted on a 24 mm BIB balloon. C) The valve positioned in the intended landing zone of the stented segment of the pulmonary homograft. D) After slow balloon inflation, the valve was inserted in its intended position with the lower part of the skirt in the stented area with the lowest minimal luminal diameter.
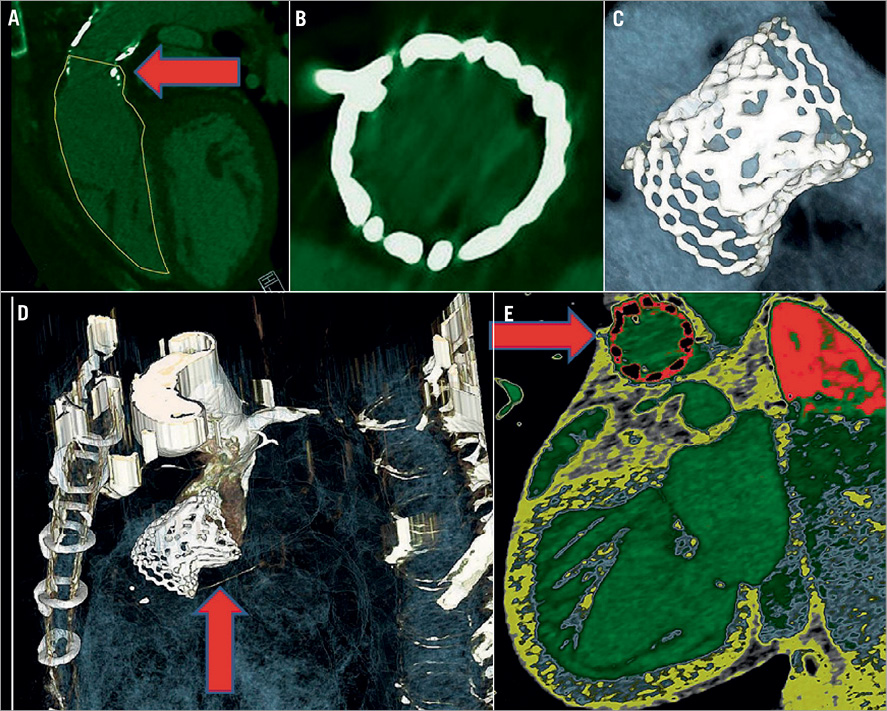
Online Figure 2. Multislice CT images. A) Demonstration of the remaining stenosis in the pulmonary conduit (arrow). B) The stent frame of the valve is circular. C) The Max™ LD stent with a SAPIEN XT implanted in the central part. D) Demonstration of the stenosis in the caudal part of the stent (arrow). E) Demonstration of the circularity and good attachment to the conduit wall excluding paravalvular leakage (arrow).

